��Ŀ����
��ʳ��Ϊԭ�Ͻ����������ۺ����õ�ijЩ��������ͼ��ʾ��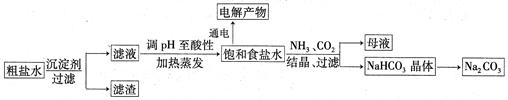
��1��Ϊ��ȥ�����е�Ca2+��Mg2+��SO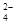 ���õ�������NaCl���壬����������Լ���
���õ�������NaCl���壬����������Լ���
| A��������NaOH��Һ�� | B��������Na2CO3��Һ�� | C����������� | D��������BaCl2��Һ�� |
��2������Һ��pH�������Գ�ȥ��������________��������Ӧ�����ӷ���ʽΪ________��
��3�����ö��Ե缫���200mL1.5mol/Lʳ��ˮ�������2minʱ���������ռ���448mL���壨��״���£���������ǰ����Һ��������䣬��������Һ��pHΪ______��
��4����������NaHCO3������ĸҺ�м��������ʯ�ң���ɻ��һ�ֿ���ѭ��ʹ�õ����ʣ��仯ѧʽ��___________��
��5�������������������й㷺��Ӧ�á�
�ٴ�������ڳ���̨���ۡ���ԭ���ǣ�������ӷ���ʽ������_____________��
�ڳ����£���ijpH=11��Na2CO3��Һ�м������ʯ���飬���˺�������ҺpH=13����Ӧǰ����Һ���뷴Ӧ�����Һ��ˮ�������
 ��OH-���ı�ֵ��_________��
��OH-���ı�ֵ��_________��
��1�� adbc��dabc (2��) ���˺������ᾧ (2��)
��2��OH-��CO32- (2��) H+ + OH- = H2O (1��) CO32- + 2H+ = CO2��+ H2O (1��)
��3��13 (2��)
��4��NH3 (1��) ��5���� CO32- + H2O HCO3- + OH-������Һ�ɳ����� (2��) �� 1010��1 (2��)
��5���� CO32- + H2O HCO3- + OH-������Һ�ɳ����� (2��) �� 1010��1 (2��)
���������������1�����ӹ����У���ʱΪ�ܽ�����ȫ����ȥ�����ó����Լ������Լӹ��������������Լ�����Ҫ�ܱ������Լ��������������˽����ʵ�鲽���Ŀ�ģ����ܷ������˳���������NaOH��Һ��Ŀ���dz�ȥMg2�������������BaCl2��Һ��Ϊ�˳�ȥSO42-�����������Na2CO3��Һ��Ϊ�˳�ȥCa2���������Ba2����Ȼ����й��ˣ���ȥMg(OH)2��BaSO4��CaCO3��BaCO3�������Ȼ������������ᣬ��ȥ�����Na2CO3�͵�����Һ��pH��������������ȷ��ʵ�����˳��Ϊadbc��dabc����ȱ�ٵIJ��������ǹ��˺������ᾧ��
��2��������NaOH��Na2CO3��Ӧ�����ӷ���ʽΪ��H+ + OH- = H2O��CO32- + 2H+ = CO2��+ H2O��
��3�������Ȼ�����Һ��Ӧ�����ӷ���ʽΪ��2Cl?+2H2O  2OH?+H2��+Cl2�������ܷ�Ӧ�������Ϲ��ռ���0.448L��0.02 mol����ʱ�������ɵ���������������0.01mol�����������������Ƶ����ʵ�����0.02mol�������������Ƶ�Ũ��C=0.02mol��0.2L=0.1mol?L?1,����pH=13��
2OH?+H2��+Cl2�������ܷ�Ӧ�������Ϲ��ռ���0.448L��0.02 mol����ʱ�������ɵ���������������0.01mol�����������������Ƶ����ʵ�����0.02mol�������������Ƶ�Ũ��C=0.02mol��0.2L=0.1mol?L?1,����pH=13��
��4�������NaHCO3������ĸҺ��NH4Cl�����������ʯ�ң�NH4Cl��Ca(OH)2��Ӧ��õĿ���ѭ��ʹ�õ�����ΪNH3��
��5����Na2CO3Ϊǿ�������Σ�ˮ���Լ��ԣ����ӷ���ʽΪ��CO32- + H2O 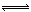 HCO3- + OH-��������Һ�ɳ����ۡ�
HCO3- + OH-��������Һ�ɳ����ۡ�
�ڷ�Ӧǰ����Һ��ˮ�������c��OH-��=1.0��10-14��10-11=1.0��10-3mol/L����Ӧ����ҺΪpH=13��ˮ�������c��OH-��=1.0��10-13mol/L�����Ա�ֵΪ1.0��10-3mol?L?1��1.0��10-13mol?L?1=1010��1��
���㣺���⿼����ε��ᴿ�����ӷ���ʽ����д�����ԭ�������Ӻ����ʵ��жϡ�����Ũ�Ⱥ�pH�ļ��㡣

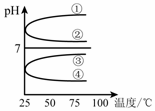
ˮ�����ؽ���Ǧ����Ⱦ���ⱸ�ܹ�ע��ˮ��Һ��Ǧ�Ĵ�����̬��Ҫ��Pb2����Pb(OH)����Pb(OH)2��Pb(OH)3-��Pb(OH)42-������̬��Ũ�ȷ���������ҺpH�仯�Ĺ�ϵ����ͼ��ʾ�� 
(1)Pb(NO3)2��Һ�У� ________2(�>����������<��)��������Һ�е����Ȼ����Һ��
________2(�>����������<��)��������Һ�е����Ȼ����Һ�� ���ӣ����ܵ�ԭ����________________________________��
���ӣ����ܵ�ԭ����________________________________��
(2)��Pb(NO3)2��Һ�е���ϡNaOH��Һ��pH��8ʱ��Һ�д��ڵ�������(Na������)��__________��pH��9ʱ��Ҫ��Ӧ�����ӷ���ʽΪ_______________________��
(3)ij�������Ʊ���һ��������Ǧ��������Чȥ��ˮ�еĺ���Ǧ��ʵ�������±���
| ���� | Pb2�� | Ca2�� | Fe3�� | Mn2�� | Cl�� |
| ����ǰŨ��/(mg��L��1) | 0.100 | 29.8 | 0.120 | 0.087 | 51.9 |
| ������Ũ��/(mg��L��1) | 0.004 | 22.6 | 0.040 | 0.053 | 49.8 |
(4)�������Ǧ��(��EH��ʾ)��Ǧ��Ҫ�����ķ�Ӧ������Ϊ��2EH(s)��Pb2��
 E2Pb(s)��2H������Ǧ�������pH��ΧΪ( )
E2Pb(s)��2H������Ǧ�������pH��ΧΪ( )A��4��5 B��6��7 C��9��10 D��11��12
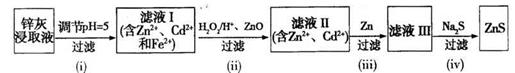
��ʽ̼��ͭ��һ����;�㷺�Ļ���ԭ�ϡ���ҵ�Ͽ������Կ�ʴ��Һ����Ҫ�ɷ���Cu2+��Fe2+��Fe3+��H +��Cl-���Ʊ������Ʊ��������£�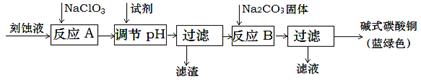
Cu2+��Fe2+��Fe3+���ɳ�����pH���£�
| ���� | Cu(OH)2 | Fe (OH)2 | Fe (OH)3 |
| ��ʼ����pH | 4.2 | 5.8 | 1.2 |
| ��ȫ����pH | 6.7 | 8.3 | 3.2 |
��1�������Ƶ������� ����������Ҫ�ɷ��� ��д��ѧʽ����
��2�����ڷ�ӦA����Һ��pH��ΧӦΪ ������ѡ����Լ��� ������ţ���
a����ˮ b��ϡ���� c���������� d��̼��ͭ
��3����ӦB���¶�����ߣ�����������ɫ��Ʒ�п��ܻ���ֵ������� ����д��ѧʽ��
��4�����˵õ��IJ�Ʒϴ��ʱ������жϲ�Ʒ�Ѿ�ϴ���� ��
��5����Na2CO3��Һ���뵽һ����CuCl2��Һ�еõ�������
�� ������ֻ��CuCO3������Ӧ�����ӷ���ʽΪ ��
�� ������ֻ��Cu(OH)2������Ӧ�����ӷ���ʽ��ʾ����� ��
�� ������Cu(OH)2��CuCO3�Ļ�������ʽ�Σ�����˵�� ��
��6����ʽ̼��ͭ��ɿɱ�ʾΪ��aCuCO3?bCu(OH)2?cH2O����ͨ�����з����ⶨ����ɡ��������£�
�� ������Ʒ���� ���·ֽ⣻�� ���CO2���������� ���ˮ�������������� ����CuO��
��������ⶨ�����������ۡ� ��
A��B��C��D������������ˮ����ȫ���룬��������������±���
| ������ | Na+��Al3+��Ba2+��H+��NH4+ |
| ������ | SO42-��OH-��CO32-��Cl- |
�ֽ�������ʵ�飺
������A��Һ��B��Һ��Ϲ��ȿ����ɳ����ʹ̼�����ζ���壻
������A��Һ��C��Һ��Ͽ����ɳ����ң�
��A��Һ��B��Һ�����ܽ�����ң����������ܽ�����ס�
��ش�
��1��A�Ļ�ѧʽΪ_________������ʱ����pH��ȵ�A��Һ��D��Һ�ֱ�ϡ��10����pH�ֱ��Ϊa��b����a _______b(�>������=����<������
��2����������C��Һ�����գ�������ù���Ϊ_______ (�ѧʽ����
��3��C��Һ��D��Һ��Ӧ�����ӷ���ʽΪ_______
��4����B��Һ����μ��������������ʵ���Ũ�ȵ�NaOH��Һ���μӹ�����ˮ�ĵ���ƽ�⽫_______ (������������������ƶ�������������Һ�и�����Ũ���ɴ�С��˳��Ϊ____________________
��5����֪������Ksp=x����0.03mol��L-1��A��Һ��0.01mol��L-1��B��Һ�������ϣ������Һ��������ӵ�Ũ��Ϊ_______ (�ú�x�Ĵ���ʽ��ʾ����Ϻ���Һ����仯���Բ��ƣ���
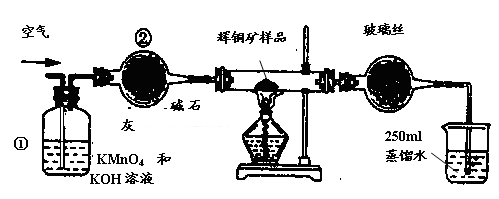
��ҵ��Ϊ�˲ⶨ��ͭ����Ҫ�ɷ���Cu2S����Cu2S�������������������ͼװ�á�ʵ��ʱ�����²��������
| A������ȫ��������ʹ���Ϊ��ͼװ�ã������װ�õ������ԡ� |
| B����ȡ��ϸ�Ļ�ͭ����Ʒ1.000g�� |
| C���������õ���ƷС�ĵط���Ӳ�ʲ������С� |
| D����ÿ����1L�����ʹ�������� |
F. ��ȡ25.00ml��SO2��ˮ��Һ��250ml��ƿ�У���0.0100mol/L KMnO4����Һ�ζ����յ㡣���������������ظ��ζ�2��3�Ρ�
�Իش��������⣺
��1��װ�âٵ�������_________________��װ�âڵ�������____________________��
��2���ٶ���ͭ���е���ȫ��ת��ΪSO2������ȫ����ˮ���գ������F����������Ӧ�Ļ�ѧ����ʽΪ ��������_______________________________������ʱ���жϵζ��Ѿ��ﵽ�յ㡣
��3��������F�ĵζ�������±���ʾ�����ͭ����Ʒ��Cu2S������������_________��
| �ζ� ���� | ������Һ�� ���/mL | ����Һ����� | |
| �ζ�ǰ�̶�/mL | �ζ���̶�/mL | ||
| 1 | 25.00 | 1.04 | 21.03 |
| 2 | 25.00 | 1.98 | 21.99 |
| 3 | 25.00 | 3.20 | 21.24 |
��4���������������һ�����Ե�ȱ��Ӱ���˲ⶨ����������ڲ���ʧ������Ϊ�� ��дһ�ּȿɣ���
��5����֪�ڳ�����FeS �� Ksp�� 6 . 25 �� 10 ��18, H2S ������Һ�� c (H������ c (S2����֮��������¹�ϵ�� c2 (H��) ��c��S2��) =" 1" . 0��10��22���ڸ��¶��£������� FeS Ͷ�����ⱥ����Һ�У���ʹ��Һ��c��Fe2+��Ϊ lmol/L��Ӧ������Һ��c��Hʮ��Ϊ__________������
 ��
��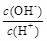 ��c(H+)��c(OH-)�ij˻� ��OH-�����ʵ���
��c(H+)��c(OH-)�ij˻� ��OH-�����ʵ���