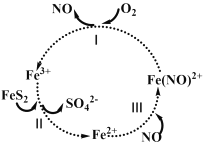
��Ŀ����
����Ŀ��ʵ������ 500��ʱ�����������������������[(NH4)2Fe(SO4)2]���ֽ���ȫ��ȷ���ֽ����ɷֵ�װ����ͼ��ʾ (��֪�ֽ�Ĺ����������� FeO��Fe2O3 �� Fe3O4�������������� NH3��N2��H2O��SO3 �� SO2)������˵���� ȷ����( )
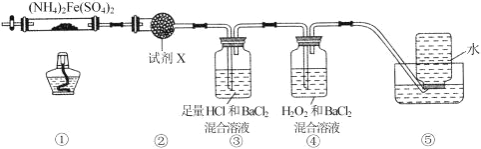
A. ȡ���й����������ϡ���ᷴӦ���μ� KSCN����Һ��죬�������һ��Ϊ Fe2O3
B. װ�â����ڼ���ֽ�������Ƿ���ˮ�������ɣ��Լ� X ���ѡ�ü�ʯ��
C. װ�â����ڼ���ֽ�������Ƿ��� SO3 �������ɲ���ȥ SO3 �� NH3
D. װ�â����ڼ���ֽ�������Ƿ��� SO2 �������ɣ�װ�â������ռ����ɵ� NH3 �� N2
���𰸡�C
��������
A��Fe3O4�к����������������Ӻ�һ�����������ӣ�ȡ���й����������ϡ���ᷴӦ���μ� KSCN����Һ��죬����������Ϊ Fe2O3��Fe3O4�������߾��У���A����
B��װ�â����ڼ���ֽ�������Ƿ���ˮ�������ɣ��Լ� X ���ѡ����ˮ����ͭ����B����
C��װ�â������������ɫ���������ɫ����Ϊ���ᱵ����֤������ SO3 ���壬�����Գ�ȥ SO3 ��װ�â��������ᣬ��˿��Գ�ȥ NH3����C��ȷ��
D��NH3 ��������ˮ����������ˮ���ռ����ڰ���������װ��ʱ�Ѿ�����ȥ����ˢ������ռ����ɵ�N2����D����
����������������ȷ��ΪC��

 ��У����ϵ�д�
��У����ϵ�д�