��Ŀ����
��15�֣���ͭ����Ҫ��2CuCO3��Cu(OH)2������������Fe��Si�Ļ������ҵ������ͭ��Ϊԭ���Ʊ�Cu��CaCO3�������ж��֡�
��1����ͭ���뽹̿���ȿ�������ͭ��������̼��ˮ��д���÷�Ӧ�Ļ�ѧ����ʽ��______________________________________________________________��
���巽���������£�
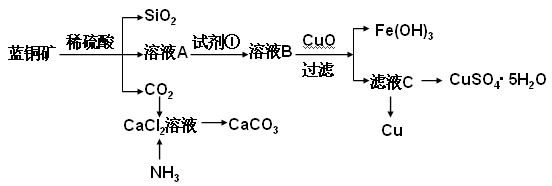
��ش��������⣺
��2����ҺA�Ľ���������Cu2+��Fe2+��Fe3+�������������Լ���ѡ������ҺA�к���Fe2+������Լ�Ϊ ������ţ���ʵ�鲽�����Լ���Ϊ ������ţ���
a��KMnO4 b��(NH4) 2S c��H2O2 d��KSCN
��3������ҺC���CuSO4��5H2O����Ҫ����������������ȴ�ᾧ�����˵Ȳ��������������� ___________________ʱֹͣ���ȡ������������������е������� ________________ ��
��4���Ʊ�CaCO3ʱ��Ӧ��CaCl2��Һ����ͨ�루���ȼ��룩 ���ѧʽ������ʵ��������а����ݳ���Ӧѡ������ װ������β��������ţ���
��5������ҺC�л��Cu�ķ����� ��д�����ֲ�ͬ��������
��6���ö��Ե缫�������ͭ��Һһ��ʱ�����2molCu(OH)2����ʹ����ͭ��Һ��ԭ����ԭ��ָ��Һ���ʳɷּ�Ũ����ԭ����ȫ��ͬ������˵������й�ת�Ƶ��� mol��
��1����ͭ���뽹̿���ȿ�������ͭ��������̼��ˮ��д���÷�Ӧ�Ļ�ѧ����ʽ��______________________________________________________________��
���巽���������£�

��ش��������⣺
��2����ҺA�Ľ���������Cu2+��Fe2+��Fe3+�������������Լ���ѡ������ҺA�к���Fe2+������Լ�Ϊ ������ţ���ʵ�鲽�����Լ���Ϊ ������ţ���
a��KMnO4 b��(NH4) 2S c��H2O2 d��KSCN
��3������ҺC���CuSO4��5H2O����Ҫ����������������ȴ�ᾧ�����˵Ȳ��������������� ___________________ʱֹͣ���ȡ������������������е������� ________________ ��
��4���Ʊ�CaCO3ʱ��Ӧ��CaCl2��Һ����ͨ�루���ȼ��룩 ���ѧʽ������ʵ��������а����ݳ���Ӧѡ������ װ������β��������ţ���

��5������ҺC�л��Cu�ķ����� ��д�����ֲ�ͬ��������
��6���ö��Ե缫�������ͭ��Һһ��ʱ�����2molCu(OH)2����ʹ����ͭ��Һ��ԭ����ԭ��ָ��Һ���ʳɷּ�Ũ����ԭ����ȫ��ͬ������˵������й�ת�Ƶ��� mol��
��1��2[2CuCO3��Cu(OH)2]+3C 6Cu+7CO2��+2 H2O ��3�֣�
6Cu+7CO2��+2 H2O ��3�֣�
��2��a c ��2�֣���1�֣� ��3����������Ĥ����ʱ���������������Ҳ�ɣ���2�֣����������裨2�֣� ��4��NH3��1�֣� b ��1�֣� ��5��������ý���Fe������д�������Ƴ�Cu����������Ҳ�ɣ����ö��Ե缫�������ͭ��Һ�� ��2�֣� ��6�� 8 ��2�֣�
 6Cu+7CO2��+2 H2O ��3�֣�
6Cu+7CO2��+2 H2O ��3�֣� ��2��a c ��2�֣���1�֣� ��3����������Ĥ����ʱ���������������Ҳ�ɣ���2�֣����������裨2�֣� ��4��NH3��1�֣� b ��1�֣� ��5��������ý���Fe������д�������Ƴ�Cu����������Ҳ�ɣ����ö��Ե缫�������ͭ��Һ�� ��2�֣� ��6�� 8 ��2�֣�
��

��ϰ��ϵ�д�
 �żӾ���ϵ�д�
�żӾ���ϵ�д�
�����Ŀ
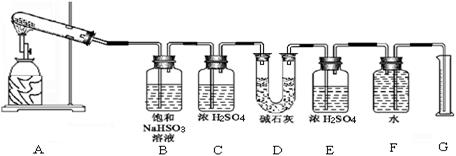
 �������ϴ����������� ��
�������ϴ����������� �� 56
56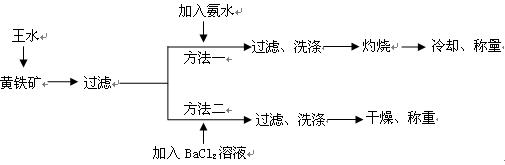
 ��(��=1.42g��cm-3)��3�����Ũ����(��=1.19g��cm-3)��϶��ɵġ�
��(��=1.42g��cm-3)��3�����Ũ����(��=1.19g��cm-3)��϶��ɵġ�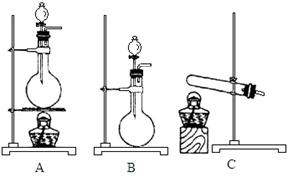

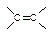 ��IBr ��
��IBr ��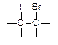
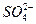 ������д�±��еĿո���ɽ������ᴿ���õ��Ȼ��ƾ����ʵ����ơ�
������д�±��еĿո���ɽ������ᴿ���õ��Ȼ��ƾ����ʵ����ơ� 1��ʵ������ܴﵽʵ��Ŀ�ĵ��� �� ��
1��ʵ������ܴﵽʵ��Ŀ�ĵ��� �� ��