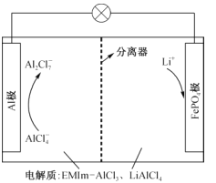
��Ŀ����
����Ŀ���ݱ�����ij���Լ״�Ϊԭ�ϣ���KOHΪ����ʵ������ֻ��Ŀɳ��ĸ�Чȼ�ϵ�أ���һ�ε������ʹ�ýϳ�ʱ�䡣��ͼ��һ���绯ѧ���̵�ʾ��ͼ����֪�׳ص��ܷ�ӦʽΪ��2CH3OH+3O2+4KOH![]() 2K2CO3+6H2O��
2K2CO3+6H2O��
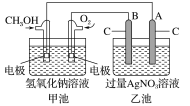
����գ�
(1)���ʱ����ȼ�ϵ�صĸ������Դ________��������
�������ĵ缫��ӦʽΪ��________________________��
(2)�ŵ�ʱ�������ĵ缫��ӦʽΪ��__________________��
(3)�ڴ˹���������ȫ��Ӧ���ҳ���A������������648 g����׳�������������O2____________L(��״��)��
(4)���ڳ��³�ѹ�£�1 g CH3OHȼ������CO2��Һ̬H2Oʱ����22.68 kJ����ʾ�÷�Ӧ���Ȼ�ѧ����ʽΪ��____________________��
���𰸡��� 4OH��-4e-=2H2O+O2�� CH3OH��8OH��-6e��=CO32-��6H2O 33.6 CH3OH(l)+![]() O2(g)=CO2(g)+2H2O(l) ��H=��725.76 kJ��mol��1
O2(g)=CO2(g)+2H2O(l) ��H=��725.76 kJ��mol��1
��������
(1)���ʱ��ԭ��ظ������Դ������������������������ԭ��Ӧ������ʧ���ӷ���������Ӧ��
(2)�ŵ�ʱ�������ϼ״�ʧ���ӷ���������Ӧ��
(3)�ҳ���A��Ϊ�����������ӵõ��ӷ�����ԭ��Ӧ������ת�Ƶ�����ȼ��������������
(4)�������ʷ�Ӧ�ų��������뷴Ӧ�����ʶ��ٳ����ȼ��㷴Ӧ�ȣ�Ȼ����д�Ȼ�ѧ����ʽ��
(1)�ٳ��ʱ��ȼ�ϵ�ظ������Դ����������
������������������ʧ���ӷ���������Ӧ���缫��ӦʽΪ��4OH--4e-=2H2O+O2����
(2)�ŵ�ʱ���״�ʧ���Ӻ����������ӷ�Ӧ����̼������Ӻ�ˮ�����Ե缫��ӦʽΪ��CH3OH��8OH--6e-=CO32-��6H2O��
(3)�ҳ���A�缫��Ag+�õ��ӷ�����ԭ��Ӧ�����ҳ���A�缫����������648g��n(Ag)=![]() =6mol�������ͬһ�պϻ�·�е���ת����Ŀ��ȿ�֪���ڼ׳�������������O2���V(O2)=
=6mol�������ͬһ�պϻ�·�е���ת����Ŀ��ȿ�֪���ڼ׳�������������O2���V(O2)=![]() 33.6L��
33.6L��
(4)���ڳ��³�ѹ�£�1g CH3OHȼ������CO2��Һ̬H2Oʱ����22.68 kJ������1mol�״���������32g������1mol CH3OHȼ������CO2��Һ̬H2Oʱ����22.68 kJ��32=725.76 kJ�����Ա�ʾ�÷�Ӧ���Ȼ�ѧ����ʽΪ��CH3OH(l)+![]() O2(g)=CO2(g)+2H2O(l) ��H=��725.76 kJ��mol��1��
O2(g)=CO2(g)+2H2O(l) ��H=��725.76 kJ��mol��1��