��Ŀ����
�ֶ���������Ȼ����֮һ������Ҫ�ɷ�Ϊˮ�������������ˮ����(���ǻ�������)�ͼ״���ԭ����Ũ��������������á�
ʵ�鲽�裺
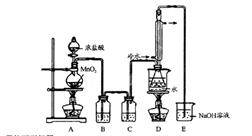
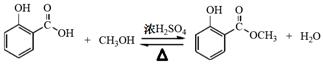
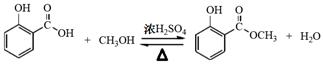
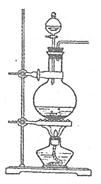
����ͼ����100 mL������ƿ�з���6.9 g (0.05 mol)ˮ�����24 g(30 mL��0.75 mol)�״����������м���Լ10 mL�ױ�,��С�ĵؼ���8 mLŨ���ᣬҡ�����ȣ�����1��2����ʯ��װ�ϻ�����������ʯ�����ϱ���85��95�棬���Ȼ���1.5��2Сʱ��
��װ����ȴ������״�������ƿ�м���50 mL��ˮ��Ȼ��ת������Һ©�����ֳ��²�����ȥ�ϲ�ˮ�㣬�л����ٵ����Һ©���У�������50 mL5��NaHCO3��Һϴ1��(��Һ��������)��30 mLˮϴһ��(��������²�)���л���õ�������
���������������ռ�221�桫224�����֡���Ʒ��0.5 g��ˮCaCl2�������ء�
��������������

�����������Ϣ�ش��������⣺
��1���Ʊ������͵Ļ�ѧ����ʽΪ______________��
��2���Ʊ�������ʱ������ʵļ��ȷ�����_______________��ʵ���м���ױ�����ˮ����Ŀ����____________________��
��3����Ӧ������ȴ����״��IJ����ǣ�___________________;
��4�����ᴿ�ֲ�Ʒ�Ĺ����У���̼��������Һϴ����Ҫ��ȥ��������____________����
��������������Һ�Ƿ����____________(����ԡ������ԡ�)����ԭ����________________��
��5�������Ʒ���Ƿ���ˮ����Ļ�ѧ������__________________�����ճƵò�Ʒ������Ϊ4.2 g��������ˮ��������IJ���Ϊ___________(������λ��Ч����)��
ʵ�鲽�裺
����ͼ����100 mL������ƿ�з���6.9 g (0.05 mol)ˮ�����24 g(30 mL��0.75 mol)�״����������м���Լ10 mL�ױ�,��С�ĵؼ���8 mLŨ���ᣬҡ�����ȣ�����1��2����ʯ��װ�ϻ�����������ʯ�����ϱ���85��95�棬���Ȼ���1.5��2Сʱ��

��װ����ȴ������״�������ƿ�м���50 mL��ˮ��Ȼ��ת������Һ©�����ֳ��²�����ȥ�ϲ�ˮ�㣬�л����ٵ����Һ©���У�������50 mL5��NaHCO3��Һϴ1��(��Һ��������)��30 mLˮϴһ��(��������²�)���л���õ�������
���������������ռ�221�桫224�����֡���Ʒ��0.5 g��ˮCaCl2�������ء�
��������������

�����������Ϣ�ش��������⣺
��1���Ʊ������͵Ļ�ѧ����ʽΪ______________��
��2���Ʊ�������ʱ������ʵļ��ȷ�����_______________��ʵ���м���ױ�����ˮ����Ŀ����____________________��
��3����Ӧ������ȴ����״��IJ����ǣ�___________________;
��4�����ᴿ�ֲ�Ʒ�Ĺ����У���̼��������Һϴ����Ҫ��ȥ��������____________����
��������������Һ�Ƿ����____________(����ԡ������ԡ�)����ԭ����________________��
��5�������Ʒ���Ƿ���ˮ����Ļ�ѧ������__________________�����ճƵò�Ʒ������Ϊ4.2 g��������ˮ��������IJ���Ϊ___________(������λ��Ч����)��
��1��
 (2��)��
(2��)��
��2��ˮԡ����(1��) ��Ӧ������ˮ�ӷ�Ӧ��ϵ�з��뿪����ʹ��ƽ�������ƶ���ͬʱ���Լ��ټ״����������Ӷ���߷�Ӧ�IJ��� (2��)
��3������(1��)
��4��ˮ����(1��) ������(1��) ������������Һ��ˮ�����������ˮ��(1��)
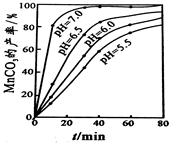
��5��ȡ�����������Թ��У�������ˮ�ܽ⣬�μ�NaHCO3��Һ����������ɫ������ˮ����(�𰸺�������)(2��) 55��(2��)
 (2��)��
(2��)����2��ˮԡ����(1��) ��Ӧ������ˮ�ӷ�Ӧ��ϵ�з��뿪����ʹ��ƽ�������ƶ���ͬʱ���Լ��ټ״����������Ӷ���߷�Ӧ�IJ��� (2��)
��3������(1��)
��4��ˮ����(1��) ������(1��) ������������Һ��ˮ�����������ˮ��(1��)
��5��ȡ�����������Թ��У�������ˮ�ܽ⣬�μ�NaHCO3��Һ����������ɫ������ˮ����(�𰸺�������)(2��) 55��(2��)
�����������1���Ʊ������͵Ļ�ѧ����ʽΪ
 (2��)
(2��)��2���Ʊ�������ʱ��85��95������ʵļ��ȷ�����ˮԡ���ȣ�ʵ���м���ױ�����ˮ����Ŀ���Ƿ�Ӧ������ˮ�ӷ�Ӧ��ϵ�з��뿪����ʹ��ƽ�������ƶ���ͬʱ���Լ��ټ״����������Ӷ���߷�Ӧ�IJ��ʣ�
��3����Ϊ�״��ķе�ͣ�����״��IJ���������
��4����̼��������Һϴ����Ҫ��ȥ����������������ˮ���NaOH��������ϴ�Ӽ�����Ϊ������������Һ��ˮ�����������ˮ�⣻
��5�������Ʒ���Ƿ���ˮ����Ļ�ѧ������ȡ�����������Թ��У�������ˮ�ܽ⣬�μ�NaHCO3��Һ����������ɫ������ˮ����(�𰸲�Ψһ)��6.9 g (0.05 mol)ˮ����Ӧ�õ�0.05molˮ�����������7.6g����Ʒ������Ϊ4.2 g��������ˮ��������IJ���Ϊ55����

��ϰ��ϵ�д�
�����Ŀ


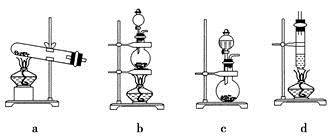
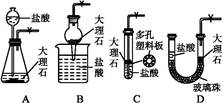
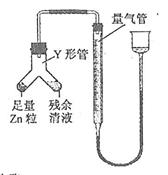
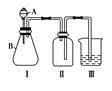 (4)����ȤС��ͬѧ��ͬ�������ͼ��ʾ��ʵ��װ�ã�����װ�â���ȡ���壬��ش��������⣺
(4)����ȤС��ͬѧ��ͬ�������ͼ��ʾ��ʵ��װ�ã�����װ�â���ȡ���壬��ش��������⣺
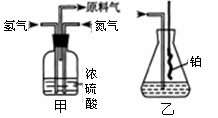
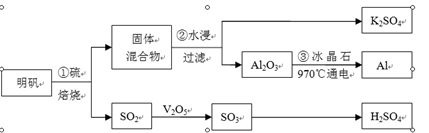
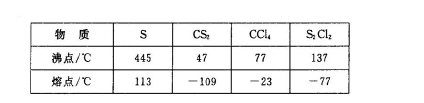
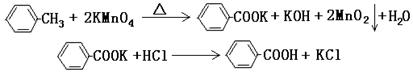
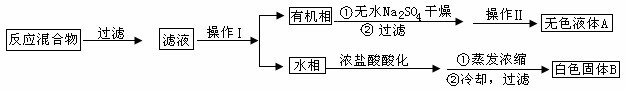
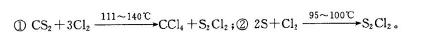 ��֪��S2Cl2����Ԫ����+1�ۣ�����ʽ��
��֪��S2Cl2����Ԫ����+1�ۣ�����ʽ��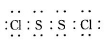 �������ȶ�����ˮ�������绯��Ӧ (һ������Ԫ�ؼ�̬���ߣ�һ���ֽ���)����Ӧ�漰�ļ������ʵ��۷е����£�
�������ȶ�����ˮ�������绯��Ӧ (һ������Ԫ�ؼ�̬���ߣ�һ���ֽ���)����Ӧ�漰�ļ������ʵ��۷е����£�