��Ŀ����
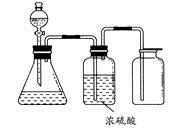
ʵ���ҳ���MnO2��Ũ���ᷴӦ�Ʊ�Cl2������װ������ͼ��ʾ����
(1)�Ʊ�ʵ�鿪ʼʱ���ȼ��װ�������ԣ��������IJ��������ǣߣ�����ţ�
A.����ƿ�м���MnO2��ĩ
B.����
C.����ƿ�м���Ũ����
(2)�Ʊ���Ӧ��������Ũ���½���ֹͣ��Ϊ�ⶨ��Ӧ����Һ�������Ũ�ȣ�̽��С��ͬѧ�������ʵ�鷽����
������������AgNO3��Һ��Ӧ���������ɵ�AgCl������
�ҷ�������������к͵ζ����ⶨ��
������������֪��CaCO3����������Ӧ������ʣ���CaCO3������
��������������Zn ��Ӧ���������ɵ�H2�����
�̶����������жϺ�ʵ�飺
�� ����������������� ��
�� �����ҷ���ʵ�飺ȷ��ȡ������Һϡ��һ����������Ϊ������
a.��ȡ����20.00 mL����0 . 1000 mol��L-1 NaOH����Һ�ζ�������22.00mL���ôεζ��������������Ũ��Ϊ mol��L-1
b.ƽ�еζ�����ʵ������
�� �жϱ�������ʵ���� ���ƫ����ƫС����ȷ������
����֪��Ksp��CaCO3 ) = 2.8��10-9��Ksp��MnCO3 ) = 2.3��10-11
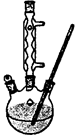
�� ���ж�����ʵ�飺װ������ͼ��ʾ���г���������ȥ����
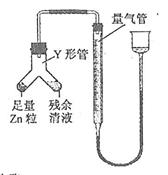
(i) ʹY�ι��еIJ�����Һ��п����Ӧ����ȷ�����ǽ� ת�Ƶ� �С�
(ii)��Ӧ��ϣ�ÿ���1 ���Ӷ�ȡ������������������μ�С��ֱ�����䡣���������μ�С��ԭ���� ���ų�������ʵ�������Ӱ�����أ���

(1)�Ʊ�ʵ�鿪ʼʱ���ȼ��װ�������ԣ��������IJ��������ǣߣ�����ţ�
A.����ƿ�м���MnO2��ĩ
B.����
C.����ƿ�м���Ũ����
(2)�Ʊ���Ӧ��������Ũ���½���ֹͣ��Ϊ�ⶨ��Ӧ����Һ�������Ũ�ȣ�̽��С��ͬѧ�������ʵ�鷽����
������������AgNO3��Һ��Ӧ���������ɵ�AgCl������
�ҷ�������������к͵ζ����ⶨ��
������������֪��CaCO3����������Ӧ������ʣ���CaCO3������
��������������Zn ��Ӧ���������ɵ�H2�����
�̶����������жϺ�ʵ�飺
�� ����������������� ��
�� �����ҷ���ʵ�飺ȷ��ȡ������Һϡ��һ����������Ϊ������
a.��ȡ����20.00 mL����0 . 1000 mol��L-1 NaOH����Һ�ζ�������22.00mL���ôεζ��������������Ũ��Ϊ mol��L-1
b.ƽ�еζ�����ʵ������
�� �жϱ�������ʵ���� ���ƫ����ƫС����ȷ������
����֪��Ksp��CaCO3 ) = 2.8��10-9��Ksp��MnCO3 ) = 2.3��10-11
�� ���ж�����ʵ�飺װ������ͼ��ʾ���г���������ȥ����

(i) ʹY�ι��еIJ�����Һ��п����Ӧ����ȷ�����ǽ� ת�Ƶ� �С�
(ii)��Ӧ��ϣ�ÿ���1 ���Ӷ�ȡ������������������μ�С��ֱ�����䡣���������μ�С��ԭ���� ���ų�������ʵ�������Ӱ�����أ���
��1��ACB������д����� ��2���ٲ�����Һ�У�n(Cl-)��n(H+)(������������)
�� 0.1100 �� ƫС �� ������Zn�� ������Һ������д����� ������ װ����������δ��������
�����㶨λ�����⿼���˻�ѧʵ�鷽������������ʵ�����������۵ȣ����ڿ���ѧ����ʵ�����������ݴ���������
�� 0.1100 �� ƫС �� ������Zn�� ������Һ������д����� ������ װ����������δ��������
�����㶨λ�����⿼���˻�ѧʵ�鷽������������ʵ�����������۵ȣ����ڿ���ѧ����ʵ�����������ݴ���������
��1��ע���ҩƷʱ�ȼ������MnO2����ͨ����Һ©������Ũ���ᣬ�����ܼ��ȡ�
������˳����ACB
(2)�ٸ��ݷ�Ӧ�����ӷ���ʽ��MnO2+4H��+2Cl��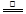 Mn2��+Cl2��+2H2O�����Կ�����Ӧ����Һ��c(Cl��)>c(H��)���ü�����õ���c(Cl��)��������(H��)��
Mn2��+Cl2��+2H2O�����Կ�����Ӧ����Һ��c(Cl��)>c(H��)���ü�����õ���c(Cl��)��������(H��)��
�ڸ���c(����)��V(����)��c(��������)��V(��������)��c(����)��c(��������)��V(��������)/ V(����)="22.00mL��0.1000" mol��L��1/20.00mL="0.1100" mol��L��1��
������KSP(MnCO3)<KSP(CaCO3)��������CaCO3Ҫת��Ϊһ����MnCO3������M(MnCO3)>M(CaCO3)��������ʣ��Ĺ����������ӣ����²�õ�c(H��)ƫС��
��Zn�����ᷴӦ���ȣ���ˣ���ȴ��������������С��
������˳����ACB
(2)�ٸ��ݷ�Ӧ�����ӷ���ʽ��MnO2+4H��+2Cl��
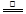 Mn2��+Cl2��+2H2O�����Կ�����Ӧ����Һ��c(Cl��)>c(H��)���ü�����õ���c(Cl��)��������(H��)��
Mn2��+Cl2��+2H2O�����Կ�����Ӧ����Һ��c(Cl��)>c(H��)���ü�����õ���c(Cl��)��������(H��)���ڸ���c(����)��V(����)��c(��������)��V(��������)��c(����)��c(��������)��V(��������)/ V(����)="22.00mL��0.1000" mol��L��1/20.00mL="0.1100" mol��L��1��
������KSP(MnCO3)<KSP(CaCO3)��������CaCO3Ҫת��Ϊһ����MnCO3������M(MnCO3)>M(CaCO3)��������ʣ��Ĺ����������ӣ����²�õ�c(H��)ƫС��
��Zn�����ᷴӦ���ȣ���ˣ���ȴ��������������С��

��ϰ��ϵ�д�
 ��ҵ����ϵ�д�
��ҵ����ϵ�д� ͬ��ѧ��һ�ζ���ϵ�д�
ͬ��ѧ��һ�ζ���ϵ�д� �����ܾ�ϵ�д�
�����ܾ�ϵ�д�
�����Ŀ
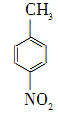 ��Na2Cr2O7��4H2SO4�D��
��Na2Cr2O7��4H2SO4�D��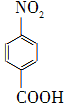 ��Na2SO4��Cr2(SO4)3��5H2O
��Na2SO4��Cr2(SO4)3��5H2O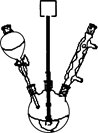

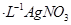 ��Һ�ζ����յ㣬����
��Һ�ζ����յ㣬����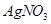 ��Һ�����ƽ��ֵΪ19��00mL��
��Һ�����ƽ��ֵΪ19��00mL��