��Ŀ����
��8�֣�ʵ��̽��������֪ʶ�IJ������γɹ��̵Ļ���;����������ijͬѧ��ɵ�̽��ʵ�鱨���һ���֣�ʵ�����ƣ�±�ص��ʵ�������ǿ���Ƚ�
ʵ��ҩƷ��KBr��Һ��KI��Һ����ˮ����ˮ����ˮ�����Ȼ�̼�����۵⻯����ֽ
��ش�
(1)��ɸ�ʵ�����õ���ʵ�������� ��
(2)CCl4��ʵ��������������� ��
(3)��ͬѧ��ʵ����Ʋ���֮���� ��
�Ľ��İ취�� ��
ʵ��ҩƷ��KBr��Һ��KI��Һ����ˮ����ˮ����ˮ�����Ȼ�̼�����۵⻯����ֽ
| ʵ�鲽�� | ʵ����� |
| ����ˮ��1 mL CCl4�������ã��۲����Ȼ�̼����ɫ | �����Դ�ǿ������˳���ȡ��塢�� |
| ��NaBr��Һ����ˮ��1 mL CCl4�������ã��۲����Ȼ�̼����ɫ | |
| ��KI��Һ����ˮ��1 mL CCl4�������ã��۲����Ȼ�̼����ɫ |
(1)��ɸ�ʵ�����õ���ʵ�������� ��
(2)CCl4��ʵ��������������� ��
(3)��ͬѧ��ʵ����Ʋ���֮���� ��
�Ľ��İ취�� ��
��ÿ��2�֣�
(1)�Թ� ��ͷ�ι� (2)��ȡ��
(3)û�бȽ�Br2��I2��������ǿ�����ѵڢ۲���Ϊ������ˮ���ڵ���KI��ֽ�ϣ��۲���ֽ�Ƿ����ɫ(��KI��Һ����ˮ��1 mL CCl4�������ã��۲����Ȼ�̼����ɫ)
(1)�Թ� ��ͷ�ι� (2)��ȡ��
(3)û�бȽ�Br2��I2��������ǿ�����ѵڢ۲���Ϊ������ˮ���ڵ���KI��ֽ�ϣ��۲���ֽ�Ƿ����ɫ(��KI��Һ����ˮ��1 mL CCl4�������ã��۲����Ȼ�̼����ɫ)
��1����ʵ������Һ�з�Ӧ����Ӧ�ﶼ��Һ�壬��Ӧ����������������֤�����飬�Ҳ���Ҫ���ȣ������õ���ʵ������ֻ���Թܺͽ�ͷ�ιܡ�
��2��ʵ�����Ǹ��ݷ�Ӧ���嵥�ʺ͵ⵥ�ʵ���ɫ���ж��Ƿ�����Ӧ���嵥�ʺ͵ⵥ��������CCl4��CCl4�ڴ˵����þ�����ȡ��͵�ĵ��ʣ���������ɫ�Ĺ۲죬����CCl4����������ȡ����
��3��ʵ�����֤�Ⱥ����ǿ����ʵ�����֤���Ⱥ͵��ǿ������ʵ����û����֤��͵��ǿ���������Dz��ܵó����۵ģ��Ľ��Ƿ��������ǣ��ѵڢ۲���Ϊ�嵥����KI��Һ��Ӧ�������Ϊ������ˮ���ڵ���KI��ֽ�ϣ��۲���ֽ�Ƿ����ɫ(��KI��Һ����ˮ��1 mL CCl4�������ã��۲����Ȼ�̼����ɫ)��
��2��ʵ�����Ǹ��ݷ�Ӧ���嵥�ʺ͵ⵥ�ʵ���ɫ���ж��Ƿ�����Ӧ���嵥�ʺ͵ⵥ��������CCl4��CCl4�ڴ˵����þ�����ȡ��͵�ĵ��ʣ���������ɫ�Ĺ۲죬����CCl4����������ȡ����
��3��ʵ�����֤�Ⱥ����ǿ����ʵ�����֤���Ⱥ͵��ǿ������ʵ����û����֤��͵��ǿ���������Dz��ܵó����۵ģ��Ľ��Ƿ��������ǣ��ѵڢ۲���Ϊ�嵥����KI��Һ��Ӧ�������Ϊ������ˮ���ڵ���KI��ֽ�ϣ��۲���ֽ�Ƿ����ɫ(��KI��Һ����ˮ��1 mL CCl4�������ã��۲����Ȼ�̼����ɫ)��

��ϰ��ϵ�д�
�����Ŀ
 ��Υ�������ﲻ����ԭ��
��Υ�������ﲻ����ԭ��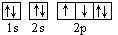 ��Υ���˺��ع���
��Υ���˺��ع��� ��
�� ��
�� ��
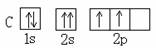
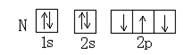
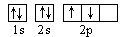
�� ������ͬ�ĵ��Ӳ�ṹ�������ƶϳ���Ԫ�ط��ű�ʾ��
������ͬ�ĵ��Ӳ�ṹ�������ƶϳ���Ԫ�ط��ű�ʾ��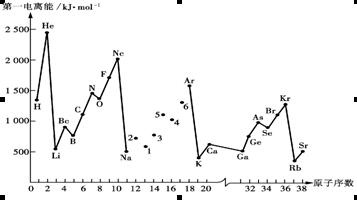
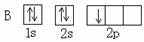
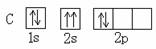 A B��
A B��