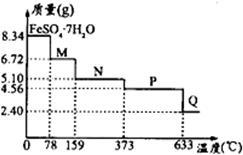
��Ŀ����
Fe�ǵؿ��к����ܷḻ��Ԫ�أ�Ҳ���������������Ԫ�ء���Ȼ��������ʯ��Ҫ�г�����ʹ������������ڸ�¯��ұ���ģ���¯�������˼�������ʯ�⣬������뽹̿��ʯ��ʯ������գ�
��д����������Ҫ�ɷֵĻ�ѧʽ�� ��
��д��������ԭ�����Ļ�ѧ����ʽ�� ��
��д����̿�ڸ�¯�������뷴Ӧ��������ѧ����ʽ�� ��
��д��CaCO3�����뷴Ӧ��������ѧ����ʽ�� ��
��д����������Ҫ�ɷֵĻ�ѧʽ�� ��
��д��������ԭ�����Ļ�ѧ����ʽ�� ��
��д����̿�ڸ�¯�������뷴Ӧ��������ѧ����ʽ�� ��
��д��CaCO3�����뷴Ӧ��������ѧ����ʽ�� ��
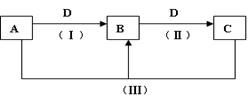
��Fe3O4 ��Fe2O3+3CO 2Fe+3CO2��Fe2O3+3C
2Fe+3CO2��Fe2O3+3C 2Fe+3CO
2Fe+3CO
��C+O2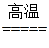 CO2 CO2+C
CO2 CO2+C 2CO
2CO
��CaCO3 CaO+CO2�� CaO+SiO2
CaO+CO2�� CaO+SiO2 CaSiO3
CaSiO3
 2Fe+3CO2��Fe2O3+3C
2Fe+3CO2��Fe2O3+3C 2Fe+3CO
2Fe+3CO��C+O2
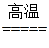 CO2 CO2+C
CO2 CO2+C 2CO
2CO��CaCO3
 CaO+CO2�� CaO+SiO2
CaO+CO2�� CaO+SiO2 CaSiO3
CaSiO3������Ҫ����������ԭ�Ϻ�������¯�з����Ļ�ѧ��Ӧ�����Ҫע�����ֳ������������������Ҫ�ɷֵIJ�ͬ���ҹ��Ǹ���������߿����г��֡�������֪ʶ��Ҳ�ǽ��������ۺϲ��ԡ���ɫ��

��ϰ��ϵ�д�
�����Ŀ
 Ϊ�˲ⶨһ��������ͭ���������ͭ������������ij��ѧ����С���������������������
Ϊ�˲ⶨһ��������ͭ���������ͭ������������ij��ѧ����С���������������������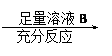
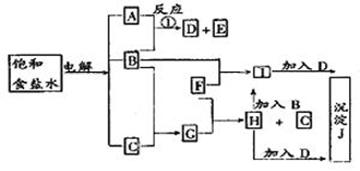
 ����ѡ����������Լ����ձ����Թܡ�����������Ͳ������ƿ���ιܡ�ҩ�ף�1mol��L-1���ᡢ2mol��L-2���ᡢ2mol��NaOH��Һ��20%KSCN��Һ��
����ѡ����������Լ����ձ����Թܡ�����������Ͳ������ƿ���ιܡ�ҩ�ף�1mol��L-1���ᡢ2mol��L-2���ᡢ2mol��NaOH��Һ��20%KSCN��Һ��