��Ŀ����
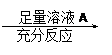 Ϊ�˲ⶨһ��������ͭ���������ͭ������������ij��ѧ����С���������������������
Ϊ�˲ⶨһ��������ͭ���������ͭ������������ij��ѧ����С���������������������������ͭ������� �ⶨ������������
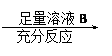 |
������ͭ������� �ⶨʣ����������
�ش��������⣺
��С���Աһ����Ϊ�������������У�����Ϊ��ʵ������ѡ�� ������ʵʩ��
��С���Աһ����Ϊ���������пɹ�ѡ�����ҺA��B�кܶ࣬����Ϊ��������������ҺA��B����ѡ�õ��� �������ţ�
A��ϡ���� B������������Һ C������ͭ��Һ
��С���ԱΪ̽��Cu2+��Al3+����Һ���Ƿ��������ת��Ϊ���������������������ʵ�飺����0.01molHNO3��0.001molCu��NO3��2��0.045molAl��NO3��3����Һ����μ���һ��Ũ�ȵ�NH3H2O��Һ��ʹ�����Һ��PHֵ�������ߣ���������Һ�����Ϊ45mLʱAl3+��ʼ��Ӧ���ɳɳ�������������Һ�����Ϊ50mLʱCu2+��ʼ��Ӧ���ɳ���������������Һ������仯��
���������ϡ���������Ksp[Al��OH��3]��1.0��10-33mol��L��4��Ksp[Cu��OH��2]��2.0��10��20mol��L��3��
��Al��OH��3��ʼ����ʱ��Һ��PHֵ��
������Һ�����ӵ�Ũ��ԼΪ1.0��10-5 mol��L��ʱ����Ϊ������ȫ����Al��OH��3������ȫʱCu��OH��2�Ƿ�������
��
��AB
�Ǣ٣����㵽��c��OH����=1.0��10-11mol��L����PH=3
�ڣ����㵽��PH=5��Cu��OH��2��ʼ����
�����㵽��PH��4.7��Al��OH��3������ȫ
Al��OH��3������ȫʱ����Cu��OH��2������
��AB
�Ǣ٣����㵽��c��OH����=1.0��10-11mol��L����PH=3
�ڣ����㵽��PH=5��Cu��OH��2��ʼ����
�����㵽��PH��4.7��Al��OH��3������ȫ
Al��OH��3������ȫʱ����Cu��OH��2������
����������ѧʵ�������²������Ȳ�����������ܵĸ����٣����Ƚϸߡ�
���������жϣ�CuSO4��Һ�����ᷢ���û���Ӧ������ͭ���ʣ����������ͭ�Ͳ���ԭ�е�ͭ�ˡ�
����������Ksp[Al��OH��3] = c3��OH����.c(Al3+)�������c��OH�����Ӷ����PH.=3
����Ksp[Cu��OH��2]��2.0��10��20mol��L��3,c(Cu2+) ��֪ �������PH=5ʱ��Cu��OH��2��ʼ����
����Ksp[Al��OH��3] = c3��OH����, c(Al3+)=1.0��10-5 mol��L�������PH��4.7��Al��OH��3������ȫ������Al��OH��3������ȫʱ����Cu��OH��2������
���������жϣ�CuSO4��Һ�����ᷢ���û���Ӧ������ͭ���ʣ����������ͭ�Ͳ���ԭ�е�ͭ�ˡ�
����������Ksp[Al��OH��3] = c3��OH����.c(Al3+)�������c��OH�����Ӷ����PH.=3
����Ksp[Cu��OH��2]��2.0��10��20mol��L��3,c(Cu2+) ��֪ �������PH=5ʱ��Cu��OH��2��ʼ����
����Ksp[Al��OH��3] = c3��OH����, c(Al3+)=1.0��10-5 mol��L�������PH��4.7��Al��OH��3������ȫ������Al��OH��3������ȫʱ����Cu��OH��2������

��ϰ��ϵ�д�
�����Ŀ
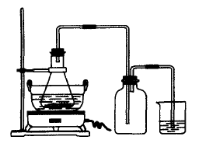
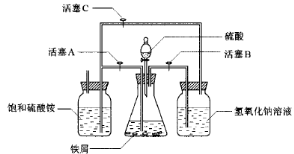
 H2SO4�����ʵ���֮��Ϊ �� ��
H2SO4�����ʵ���֮��Ϊ �� ��