��Ŀ����
17����ѧ������ѧϰ��ѧ�Ĺ��ߺͻ����������йػ�ѧ�����ʹ����ȷ���ǣ�������| A�� | ʳ�׳����Ե�ԭ���ǣ�CH3COOH+H2O=CH3COO-+H3O+ | |
| B�� | ������Һ�ʼ��Ե�ԭ���ǣ�CO32-+2H2O?H2CO3+2OH- | |
| C�� | ��������������ⱥ��ʳ��ˮ�����ӷ���ʽ��Fe+2H2O $\frac{\underline{\;���\;}}{\;}$ Fe��OH��2+H2�� | |
| D�� | ��ʾ����ȼ���ȵ��Ȼ�ѧ����ʽΪ��2H2��g��+O2��g��=2H2O��l������H=-571.6KJ•mol-1 |
���� A��������������ʲ��ֵ��룬���뷽��ʽ�ÿ���ţ�
B��̼�������Ϊ��Ԫ��������ӣ��ֲ�ˮ�⣻
C��������������ʧȥ���ӷ���������Ӧ������������������������
D��ȼ���ȹ涨�˿�ȼ������ʵ���Ϊ1mol����Ӧ�Ȼ�ѧ����ʽ�п�ȼ��Ļ�ѧ������Ϊ1��
��� �⣺A��������뷽��ʽ��CH3COOH+H2O?CH3COO-+H3O+����A����
B��̼����ֲ�ˮ�⣬�Ե�һ��Ϊ�������ӷ���ʽ��CO32-+H2O?HCO3-+OH-����B����
C����������������ⱥ��ʳ��ˮ�����ӷ���ʽ��Fe+2H2O $\frac{\underline{\;���\;}}{\;}$ Fe��OH��2+H2������C��ȷ��
D��ȼ���ȹ涨�˿�ȼ������ʵ���Ϊ1mol����Ӧ�Ȼ�ѧ����ʽ�п�ȼ��Ļ�ѧ������Ϊ1����D����
��ѡ��C��
���� ���⿼�������ӷ���ʽ����д����ȷ��Ӧʵ���ǽ���ؼ���ע������ǿ��������ˮ�����Ϊ�״��㣮

��ϰ��ϵ�д�
�����Ŀ
8������к͵ζ���������Ķ���������ѧʵ�飬�����£���50mL0.5mol•L-1HA��Һ����μ���ǿ��MOH��Һ��ͼ����ʾ���߱�ʾ�����Һ��pH�仯����������仯���Բ��ƣ�������������ȷ���ǣ�������


| A�� | ��ͼ����Ϣ��֪HAΪǿ�ᣬN���ʾ���ǡ���к� | |
| B�� | �����£�һ��Ũ�ȵ�MAϡ��Һ��pH��7 | |
| C�� | K������Ӧ����Һ������Ũ�ȵĴ�С��ϵ��c��M+����c��OH-����c��A-����c��H+�� | |
| D�� | K���Ӧ����Һ�У���Һ��pH��13��c��HA��+c��A-��=0.25mol•L-1 |
5������Ӧ���У���Ҫ�������������Ե��ǣ�������
| A�� | ���̵����кͼ��Է�ˮ | B�� | �ظ�������ھƼݼ�� | ||
| C�� | �ñ��������ȩ��֬ | D�� | �����ӹ�ǰ��������ϴ |
2��ijѧϰС���ͬѧ��ѧϰ�˻�ѧ��Ӧ�����뻯ѧƽ��֪ʶ�Է�Ӧ��aA��g��+bB��g��?cC��g��+dD��g����H����Ӧ�ص����Ӧ��ͼ��չ�������ۣ����в���ȷ���ǣ�������
| A�� | 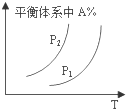 ͼ�У���P1��P2����÷�Ӧ�ڽϵ��¶����������Է����� | |
| B�� |  ͼ�У���T2��T1�����H��0��a+b=c+d | |
| C�� | 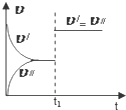 ͼ�У�v���ʾ����Ӧ���ʣ�v���ʾ�淴Ӧ���ʣ���t1ʱ�̸ı������һ����ʹ���˴��� | |
| D�� | 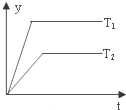 ͼ�У�����H��0���������겻���ܱ�ʾ���Ƿ�Ӧ���ת���� |
6�� �״���һ����Ҫ�Ļ���ԭ�ϣ��㷺Ӧ���ڻ���������Ҳ����ֱ������ȼ�ϣ�
�״���һ����Ҫ�Ļ���ԭ�ϣ��㷺Ӧ���ڻ���������Ҳ����ֱ������ȼ�ϣ�
��1����֪2CH3OH��l��+3O2��g��=2CO2��g��+4H2O��g����H=-1275.6kJ/mol
2CO��g��+O2��g��=2CO2��g����H=-566.0kJ/mol
H2O��g��=H2O��1����H=-44.0kJ/mol
�״�����ȫȼ��������̬ˮ���Ȼ�ѧ����ʽΪ2CH3OH��l��+O2��g��=2CO��g��+4H2O��l����H=-533.6kJ•mol-1
������ȫȼ��20g�״�����Һ̬ˮ����ʱ�ų�������Ϊ333.5kJ
��2����CO2������ȼ�ϼ״��ķ�Ӧԭ����CO2��g��+3H2��g��?CH3OH��g��+H2O��g����ijЩ��ѧ���ļ����������±���
����������Ӧ���ʱ��H=��2d+6b-3a-c-3e��kJ/mol������Ӧ��ĸ��ʾ������ֻ��ѹ����ƽ�ⳣ��K���䣨ѡ���������С�����䡱����
��3����¯���������ķ����е�CO�ɽ��л��գ�ʹ����һ�������º�H2��Ӧ�Ʊ��״���CO��g��+2H2��g��?CH3OH��g��
�����¶Ⱥ��ݻ���ͬ�������ܱ������У�����ͬ��ʽͶ�뷴Ӧ���÷�Ӧ�ﵽƽ��ʱ���й����������
���й�ϵ��ȷ����AD
A��c1=c2 B.2Q1=Q3 C.2a1=a3 D��a1+a2=1
E���÷�Ӧ������1molCH2OH����ų���Q1+Q3��kJ����
��4������һ����ɱ���ܱ������г���1molCO��2molH2��1molCH2OH���ﵽƽ��ʱ��û��������ܶ���ͬ��ͬѹ����ʼ�ܶȵ�1.6�������ƽ��ǰv��������v���棩�����������������=������
��5���״�һ�������ԣ�KOH��ȼ�ϵ������Դ��⾫����ͭ����ͼ�����ڽ�ͨ��·һ��ʱ���Cu��������3.2g����ͭ�����ʲ�����缫��Ӧ����
����д��ȼ�ϵ���еĸ�����Ӧʽ��CH3OH-6e-+8OH-=CO32-+6H2O
��ȼ�ϵ���������Ŀ����������2.8L����״̬��������O2���������20%���㣩��
 �״���һ����Ҫ�Ļ���ԭ�ϣ��㷺Ӧ���ڻ���������Ҳ����ֱ������ȼ�ϣ�
�״���һ����Ҫ�Ļ���ԭ�ϣ��㷺Ӧ���ڻ���������Ҳ����ֱ������ȼ�ϣ���1����֪2CH3OH��l��+3O2��g��=2CO2��g��+4H2O��g����H=-1275.6kJ/mol
2CO��g��+O2��g��=2CO2��g����H=-566.0kJ/mol
H2O��g��=H2O��1����H=-44.0kJ/mol
�״�����ȫȼ��������̬ˮ���Ȼ�ѧ����ʽΪ2CH3OH��l��+O2��g��=2CO��g��+4H2O��l����H=-533.6kJ•mol-1
������ȫȼ��20g�״�����Һ̬ˮ����ʱ�ų�������Ϊ333.5kJ
��2����CO2������ȼ�ϼ״��ķ�Ӧԭ����CO2��g��+3H2��g��?CH3OH��g��+H2O��g����ijЩ��ѧ���ļ����������±���
| ��ѧ�� | C-H | H-H | C-O | C=O | H-O |
| ����/kJ•mol-1 | a | b | c | d | e |
��3����¯���������ķ����е�CO�ɽ��л��գ�ʹ����һ�������º�H2��Ӧ�Ʊ��״���CO��g��+2H2��g��?CH3OH��g��
�����¶Ⱥ��ݻ���ͬ�������ܱ������У�����ͬ��ʽͶ�뷴Ӧ���÷�Ӧ�ﵽƽ��ʱ���й����������
| ���� | ��Ӧ��Ͷ����� | ��Ӧ���ת���� | CH2OH��Ũ�� | �����仯 ��Q1��Q2��Q3������0�� |
| �� | 1molCO��2molH2 | a1 | c1 | �ų�Q1kJ���� |
| �� | 1molCH3OH | a2 | c2 | �ų�Q2kJ���� |
| �� | 2molCO��4molH2 | a3 | c3 | �ų�Q3kJ���� |
A��c1=c2 B.2Q1=Q3 C.2a1=a3 D��a1+a2=1
E���÷�Ӧ������1molCH2OH����ų���Q1+Q3��kJ����
��4������һ����ɱ���ܱ������г���1molCO��2molH2��1molCH2OH���ﵽƽ��ʱ��û��������ܶ���ͬ��ͬѹ����ʼ�ܶȵ�1.6�������ƽ��ǰv��������v���棩�����������������=������
��5���״�һ�������ԣ�KOH��ȼ�ϵ������Դ��⾫����ͭ����ͼ�����ڽ�ͨ��·һ��ʱ���Cu��������3.2g����ͭ�����ʲ�����缫��Ӧ����
����д��ȼ�ϵ���еĸ�����Ӧʽ��CH3OH-6e-+8OH-=CO32-+6H2O
��ȼ�ϵ���������Ŀ����������2.8L����״̬��������O2���������20%���㣩��
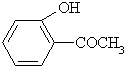 �ң�
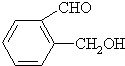
�ң�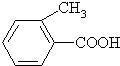 ����
����
 ij�о���ѧϰС��Ϊ̽��п�����ᷴӦ��ȡͬ������ͬ�����пƬ��ͬŨ��������������ƽ��ʵ�飺
ij�о���ѧϰС��Ϊ̽��п�����ᷴӦ��ȡͬ������ͬ�����пƬ��ͬŨ��������������ƽ��ʵ�飺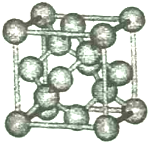 2014��12��6���й��������������ձ�������ѧ��ѧ��ȷ���������·��ֵľ��и߶Ȼ��ԵĹ軯��SiC2N��SiC3N���ӣ������о�������������ѧ�����Ǽʽ�����Ѱ����ط��ӣ��ش��������⣺
2014��12��6���й��������������ձ�������ѧ��ѧ��ȷ���������·��ֵľ��и߶Ȼ��ԵĹ軯��SiC2N��SiC3N���ӣ������о�������������ѧ�����Ǽʽ�����Ѱ����ط��ӣ��ش��������⣺
 $\stackrel{һ������}{��}$
$\stackrel{һ������}{��}$ +nH2O��
+nH2O�� ��
��