ĢāÄæÄŚČŻ
”¾ĢāÄæ”æŅŃÖŖ£ŗ298KŹ±£¬Ksp(MnS)=4.65”Į10-14£¬Ksp(MnCO3)=2.24”Į10-11”£298KŹ±£¬MnS”¢MnCO3ŌŚĖ®ÖŠ³ĮµķČܽāĘ½ŗāĒśĻßČēĶ¼ĖłŹ¾”£ĻĀĮŠĖµ·ØÕżČ·µÄŹĒ£Ø £©

A.Ķ¼ĻńÖŠeµć“¦ČÜŅŗc(Mn2+)”Ö5.0”Į10-6
B.³£ĪĀĻĀ£¬¼ÓĖ®Ļ”ŹĶXČÜŅŗæÉŹ¹dµć×ŖŅʵ½fµć
C.ĘäĖūĢõ¼ž²»±ä£¬¼ÓČČŗ¬Y¹ĢĢåµÄYČÜŅŗæÉŹ¹eµćÉżµ½fµć
D.ŌŚMnSŗĶMnCO3±„ŗĶČÜŅŗÖŠ¼ÓÉŁĮæMnCl2£¬![]() Ōö“ó
Ōö“ó
”¾“š°ø”æC
”¾½āĪö”æ
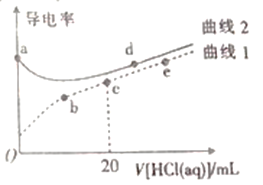
ŅŃÖŖ298KŹ±£¬Ksp(MnS)=4.65”Į10-14£¬Ksp(MnCO3)=2.24”Į10-11£¬æÉÖŖMnS½ĻÄŃČÜÓŚĖ®£¬ŌņĶ¼ÖŠXĪŖMnS”¢YĪŖMnCO3µÄČܽā¶ČĒśĻߣ¬ĒśĻßÉĻµÄµćĪŖĘ½ŗāµć£¬ŅŌ“Ė½ā“šøĆĢā”£
A. Ķ¼ĻńÖŠeµć“¦ČÜŅŗĪŖ£ŗc(Mn2+)=![]() ”Ö4.73”Į10-6£¬¹ŹA“ķĪó£»
”Ö4.73”Į10-6£¬¹ŹA“ķĪó£»
B. ³£ĪĀĻĀ£¬¼ÓĖ®Ļ”ŹĶXČÜŅŗ£¬c(Mn2+)”¢c(S2-)¼õŠ”£¬dµć×ŖŅʵ½fµć£¬c(Mn2+)Ć»±ä£¬¹ŹB“ķĪó£»
C. YĪŖMnCO3µÄĒśĻߣ¬ĘäĖūĢõ¼ž²»±ä£¬¼ÓČČŗ¬MnCO3¹ĢĢåµÄMnCO3ČÜŅŗ£¬ĪĀ¶Č±äøߣ¬ČܶȻż±ä“ó£¬c(Mn2+)ŗĶc(CO32-)±ä“ó£¬æÉŹ¹eµćÉżµ½fµć£¬¹ŹCÕżČ·£»
D. ŌŚMnSŗĶMnCO3±„ŗĶČÜŅŗÖŠ“ęŌŚ»ÆŃ§Ę½ŗā£¬MnCO3+S2-![]() MnS+CO32-£¬
MnS+CO32-£¬![]() =
=![]() £¬ČܶȻż³£ŹżÖ»ÓėĪĀ¶ČÓŠ¹Ų£¬ĖłŅŌ±ČÖµ²»±ä£¬¹ŹD“ķĪó”£
£¬ČܶȻż³£ŹżÖ»ÓėĪĀ¶ČÓŠ¹Ų£¬ĖłŅŌ±ČÖµ²»±ä£¬¹ŹD“ķĪó”£

 »ĘøŌĢģĢģĮ·æŚĖćĢāæØĻµĮŠ“š°ø
»ĘøŌĢģĢģĮ·æŚĖćĢāæØĻµĮŠ“š°ø”¾ĢāÄæ”湤ŅµÉĻÉč¼Ę½«VOSO4ÖŠµÄK2SO4”¢SiO2ŌÓÖŹ³żČ„²¢»ŲŹÕµĆµ½V2O5µÄĮ÷³ĢČēĻĀ£ŗ
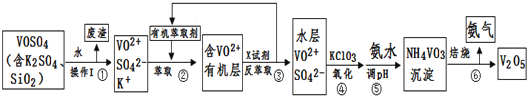
Ēė»Ų“šĻĀĮŠĪŹĢā£ŗ
£Ø1£©²½Öč¢ŁĖłµĆ·ĻŌüµÄ³É·ÖŹĒ____________Š“»ÆѧŹ½£©£¬²Ł×÷IµÄĆū³Ę______”£
£Ø2£©²½Öč¢Ś”¢¢ŪµÄ±ä»Æ¹ż³Ģæɼņ»ÆĪŖ£ØĻĀŹ½R±ķŹ¾VO2+£¬HA±ķŹ¾ÓŠ»śŻĶČ”¼Į£©£ŗ
R2(SO4)n(Ė®²ć)+ 2nHA£ØÓŠ»ś²ć£©![]() 2RAn£ØÓŠ»ś²ć£© + nH2SO4 (Ė®²ć)
2RAn£ØÓŠ»ś²ć£© + nH2SO4 (Ė®²ć)
¢ŚÖŠŻĶČ”Ź±±ŲŠė¼ÓČėŹŹĮæ¼ī£¬ĘäŌŅņŹĒ____________________________”£
¢ŪÖŠXŹŌ¼ĮĪŖ___________________”£
£Ø3£©¢ŻµÄĄė×Ó·½³ĢŹ½ĪŖ________________________”£
£Ø4£©25”ꏱ£¬Č”Ńł½ųŠŠŹŌŃé·ÖĪö£¬µĆµ½·°³ĮµķĀŹŗĶČÜŅŗpHÖ®¼ä¹ŲĻµČēĻĀ±ķ£ŗ
pH | 1£®3 | 1£®4 | 1£®5 | 1£®6 | 1£®7 | 1£®8 | 1£®9 | 2£®0 | 2£®1 |
·°³ĮµķĀŹ% | 88£®1 | 94£®8 | 96£®5 | 98£®0 | 98£®8 | 98£®8 | 96£®4 | 93£®1 | 89£®3 |
½įŗĻÉĻ±ķ£¬ŌŚŹµ¼ŹÉś²śÖŠ£¬¢ŻÖŠ¼ÓČė°±Ė®£¬µ÷½ŚČÜŅŗµÄ×ī¼ŃpHĪŖ__________£»
Čō·°³ĮµķĀŹĪŖ93£®1%Ź±²»²śÉśFe(OH)3³Įµķ£¬ŌņČÜŅŗÖŠc(Fe3+)<_____________”£
(ŅŃÖŖ£ŗ25”ꏱ£¬Ksp[Fe(OH)3]=2£®6”Į10-39)
£Ø5£©øĆ¹¤ŅÕĮ÷³ĢÖŠ£¬æÉŅŌŃ»·ĄūÓƵÄĪļÖŹÓŠ______________ŗĶ_______”£
”¾ĢāÄæ”æĮņ“śĮņĖįÄĘ£ØNa2S2O3£©æÉÓÉŃĒĮņĖįÄĘŗĶĮņ·ŪĶعż»ÆŗĻ·“Ó¦ÖʵƔ£ŅŃÖŖ£ŗNa2S2O3ŌŚĖįŠŌČÜŅŗÖŠ²»ÄÜĪČ¶Ø“ęŌŚ”£
£Ø1£©Ä³ŃŠ¾æŠ”×éÉč¼ĘĮĖÖʱøNa2S2O3”¤5H2O×°ÖĆŗĶ²æ·Ö²Ł×÷²½ÖčČēĻĀ”£
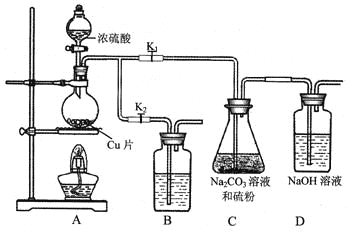
I£®“ņæŖK1£¬¹Ų±ÕK2£¬ĻņŌ²µ×ÉÕĘæÖŠ¼ÓČė×ćĮæÅØĮņĖį£¬¼ÓČČ”£
II£®CÖŠ»ģŗĻŅŗ±»ĘųĮ÷½Į¶Æ£¬·“Ó¦Ņ»¶ĪŹ±¼äŗó£¬Įņ·ŪµÄĮæÖš½„¼õÉŁ”£
µ±CÖŠČÜŅŗµÄpH½Ó½ü7Ź±¼“Ķ£Ö¹CÖŠµÄ·“Ó¦£¬Ķ£Ö¹¼ÓČČ”£
III£®¹żĀĖCÖŠµÄ»ģŗĻŅŗ”£
IV£®½«ĀĖŅŗ¼ÓČČÅØĖõ”¢ĄäČ“½į¾§”¢¹żĀĖ”¢Ļ“µÓ”¢ŗęøÉ£¬µĆµ½²śĘ·”£
¢ŁIÖŠ£¬Ō²µ×ÉÕĘæÖŠ·¢Éś·“Ó¦µÄ»Æѧ·½³ĢŹ½ŹĒ_________”£
¢ŚIIÖŠ£¬”°µ±CÖŠČÜŅŗµÄpH½Ó½ü7Ź±¼“Ķ£Ö¹CÖŠµÄ·“Ó¦”±µÄŌŅņŹĒ__________”£
”°Ķ£Ö¹CÖŠµÄ·“Ó¦”±µÄ²Ł×÷ŹĒ___________”£
¢ŪIIIÖŠ£¬”°¹żĀĖ”±ÓƵ½µÄ²£Į§ŅĒĘ÷ŹĒ£ØĢīŅĒĘ÷Ćū³Ę£©___________”£
¢Ü×°ÖĆBÖŠŹ¢·ÅµÄŹŌ¼ĮŹĒ£ØĢī»ÆѧŹ½£©________ČÜŅŗ£¬Ęä×÷ÓĆŹĒ__________”£
£Ø2£©ŅĄ¾Ż·“Ó¦2S2O32-+I2=S4O2-6+2I-£¬æÉÓĆI2µÄ±ź×¼ČÜŅŗ²ā¶Ø²śĘ·µÄ“æ¶Č”£Č”5.5g²śĘ·£¬ÅäÖĘ³É100mLČÜŅŗ”£Č”10mLČÜŅŗ£¬ŅŌµķ·ŪČÜŅŗĪŖÖøŹ¾¼Į£¬ÓĆÅضČĪŖ0.050mol/LI2µÄ±ź×¼ČÜŅŗ½ųŠŠµĪ¶Ø£¬Ļą¹ŲŹż¾Ż¼ĒĀ¼ČēĻĀ±ķĖłŹ¾”£
±ąŗÅ | 1 | 2 | 3 | 4 |
ČÜŅŗµÄĢå»ż/mL | 10.00 | 10.00 | 10.00 | 10.00 |
ĻūŗÄI2±ź×¼ČÜŅŗµÄĢå»ż/mL | 19.99 | 19.98 | 17.13 | 20.03 |
¢ŁÅŠ¶Ļ“ļµ½µĪ¶ØÖÕµćµÄĻÖĻóŹĒ______________”£
¢ŚNa2S2O3”¤5H2OŌŚ²śĘ·ÖŠµÄÖŹĮæ·ÖŹżŹĒ£Ø¼ĘĖć½į¹ū±£Įō1Ī»Š”Źż£©_________”£
£ØNa2S2O3”¤5H2OµÄŹ½ĮæĪŖ248£©