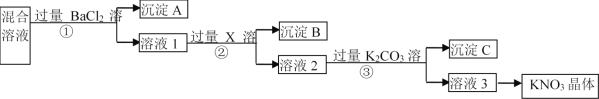
ΧβΡΩΡΎ»ί
ΓΨΧβΡΩΓΩΗυΨίΧβ“βΦΤΥψΧνΩ’ΓΘ
Θ®1Θ©‘Ύ±ξΉΦΉ¥Ωωœ¬Θ§8.5gΡ≥ΤχΧε’Φ”–ΒΡΧεΜΐΈΣ5.6LΘ§‘ρΗΟΤχΧεΒΡΡΠΕϊ÷ ΝΩ «__ΓΘ
Θ®2Θ©‘Ύ±ξΉΦΉ¥Ωωœ¬Θ§0.01molΡ≥ΤχΧεΒΡ÷ ΝΩΈΣ0.28gΘ§‘ρΗΟΤχΧεΒΡœύΕ‘Ζ÷Ή”÷ ΝΩΈΣ__Θ§ΗΟΤχΧεΒΡΟήΕ»ΈΣ__gΓΛL1ΓΘ
Θ®3Θ©‘Ύ±ξΉΦΉ¥Ωωœ¬Θ§”…COΚΆCO2Ήι≥…ΒΡΜλΚœΤχΧεΈΣ6.72LΘ§÷ ΝΩΈΣ12gΘ§¥ΥΜλΚœΈο÷–COΚΆCO2Έο÷ ΒΡΝΩ÷°±» «__Θ§CΚΆO‘≠Ή”Ηω ΐ±» «__Θ§COΒΡ÷ ΝΩΖ÷ ΐ «__ΓΘ
Θ®4Θ©œ÷”–mgΡ≥ΤχΧεΘ§Υϋ”…ΥΪ‘≠Ή”Ζ÷Ή”ΙΙ≥…Θ§ΥϋΒΡœύΕ‘Ζ÷Ή”÷ ΝΩΈΣMΓΘ»τΑΔΖϋΌΛΒ¬¬ό≥Θ ΐ”ΟNA±μ ΨΘ§‘ρΘΚΗΟΤχΧεΒΡΈο÷ ΒΡΝΩΈΣ__molΘΜΗΟΤχΧεΥυΚ§‘≠Ή”Ήή ΐΈΣ__ΗωΘΜΗΟΤχΧε‘Ύ±ξΉΦΉ¥Ωωœ¬ΒΡΧεΜΐΈΣ__LΘΜΗΟΤχΧε»ή”ΎΥ°Κσ–Έ≥…1L»ή“ΚΘ®ΗΟΤχΧε≤Μ”κΥ°Ζ¥”ΠΘ©Θ§ΤδΈο÷ ΒΡΝΩ≈®Ε»ΈΣ__molΓΛLΘ≠1ΓΘ
ΓΨ¥πΑΗΓΩ34g/mol 28 1.25 1ΘΚ3 4ΘΚ7 17.5% ![]()
![]()
![]()
![]()
ΓΨΫβΈωΓΩ
Θ®1Θ©ΗΟΤχΧεΒΡΡΠΕϊ÷ ΝΩ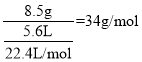 Θ§
Θ§
Ι ¥πΑΗΈΣΘΚ34g/molΘΜ
Θ®2Θ©0.01molΡ≥ΤχΧεΒΡ÷ ΝΩΈΣ0.28gΘ§ΡΠΕϊ÷ ΝΩΈΣ![]() Θ§Ι œύΕ‘Ζ÷Ή”÷ ΝΩΈΣ28ΘΜ±ξΩωœ¬ΧεΜΐ «0.224LΘ§Ι ΟήΕ»ΈΣ
Θ§Ι œύΕ‘Ζ÷Ή”÷ ΝΩΈΣ28ΘΜ±ξΩωœ¬ΧεΜΐ «0.224LΘ§Ι ΟήΕ»ΈΣ![]() Θ§
Θ§
¥πΑΗΈΣΘΚ28ΘΜ1.25;
Θ®3Θ©…η![]() ΒΡΈο÷ ΒΡΝΩΈΣxmolΘ§
ΒΡΈο÷ ΒΡΝΩΈΣxmolΘ§![]() ΒΡΈο÷ ΒΡΝΩΈΣymolΘ§±ξΉΦΉ¥Ωωœ¬ΉήΧεΜΐ6.72LΘ§Φ¥ΉήΈο÷ ΒΡΝΩΈΣ0.3molΘ§Ι x+y=0.3Θ§Ήή÷ ΝΩΈΣ12gΘ§Ι 28x+44y=12Θ§ΫβΒΟx=0.075Θ§y=0.225Θ§Ι
ΒΡΈο÷ ΒΡΝΩΈΣymolΘ§±ξΉΦΉ¥Ωωœ¬ΉήΧεΜΐ6.72LΘ§Φ¥ΉήΈο÷ ΒΡΝΩΈΣ0.3molΘ§Ι x+y=0.3Θ§Ήή÷ ΝΩΈΣ12gΘ§Ι 28x+44y=12Θ§ΫβΒΟx=0.075Θ§y=0.225Θ§Ι ![]() ΚΆ
ΚΆ![]() Έο÷ ΒΡΝΩ÷°±» «ΈΣ1:3Θ§
Έο÷ ΒΡΝΩ÷°±» «ΈΣ1:3Θ§![]() ΚΆ
ΚΆ![]() ‘≠Ή”Ηω ΐ±» «4:7Θ§COΒΡ÷ ΝΩΖ÷ ΐΈΣ
‘≠Ή”Ηω ΐ±» «4:7Θ§COΒΡ÷ ΝΩΖ÷ ΐΈΣ![]() Θ§
Θ§
Ι ¥πΑΗΈΣΘΚ1ΘΚ3 ΘΜ4ΘΚ7ΘΜ 17.5%ΘΜ
Θ®4Θ©ΗΟΤχΧεΒΡΈο÷ ΒΡΝΩΈΣ![]() molΘΜ“ρΈΣΗΟΤχΧεΈΣΥΪ‘≠Ή”Ζ÷Ή”Θ§Ι ΥυΚ§‘≠Ή”Ήή ΐΈΣ
molΘΜ“ρΈΣΗΟΤχΧεΈΣΥΪ‘≠Ή”Ζ÷Ή”Θ§Ι ΥυΚ§‘≠Ή”Ήή ΐΈΣ![]() ΗωΘΜΗΟΤχΧε‘Ύ±ξΉΦΉ¥Ωωœ¬ΒΡΧεΜΐΈΣ
ΗωΘΜΗΟΤχΧε‘Ύ±ξΉΦΉ¥Ωωœ¬ΒΡΧεΜΐΈΣ![]() LΘΜΗΟΤχΧε»ή”ΎΥ°Κσ–Έ≥…1L»ή“ΚΘ§ΤδΈο÷ ΒΡΝΩ≈®Ε»ΈΣ
LΘΜΗΟΤχΧε»ή”ΎΥ°Κσ–Έ≥…1L»ή“ΚΘ§ΤδΈο÷ ΒΡΝΩ≈®Ε»ΈΣ![]() molΓΛLΘ≠1Θ§
molΓΛLΘ≠1Θ§
Ι ¥πΑΗΈΣΘΚ![]() ΘΜ
ΘΜ![]() ΘΜ
ΘΜ![]() ΘΜ
ΘΜ![]()

 Οϊ–ΘΩΈΧΟœΒΝ–¥πΑΗ
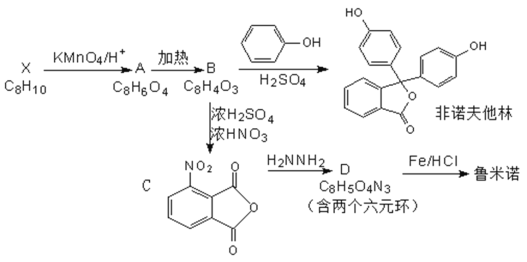
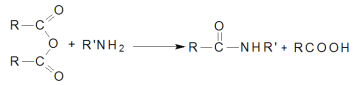
Οϊ–ΘΩΈΧΟœΒΝ–¥πΑΗΓΨΧβΡΩΓΩ”…CH3CH2CH2Br÷Τ±ΗCH3CH(OH)CH2OHΘ§“ά¥ΈΖΔ…ζΒΡΖ¥”Πάύ–ΆΚΆΖ¥”ΠΧθΦΰΕΦ’ΐ»ΖΒΡ «
―Γœν | Ζ¥”Πάύ–Ά | Ζ¥”ΠΧθΦΰ |
A | Φ”≥…ΓΔ»Γ¥ζΓΔœϊ»Ξ | KOH¥Φ»ή“Κ/Φ”»»ΓΔKOHΥ°»ή“Κ/Φ”»»ΓΔ≥ΘΈ¬ |
B | œϊ»ΞΓΔΦ”≥…ΓΔ»Γ¥ζ | NaOH¥Φ»ή“Κ/Φ”»»ΓΔ≥ΘΈ¬ΓΔKOHΥ°»ή“Κ/Φ”»» |
C | ―θΜ·ΓΔ»Γ¥ζΓΔœϊ»Ξ | Φ”»»ΓΔKOH¥Φ»ή“Κ/Φ”»»ΓΔKOHΥ°»ή“Κ/Φ”»» |
D | œϊ»ΞΓΔΦ”≥…ΓΔΥ°Ϋβ | NaOHΥ°»ή“Κ/Φ”»»ΓΔ≥ΘΈ¬ΓΔNaOH¥Φ»ή“Κ/Φ”»» |
A. A B. B C. C D. D