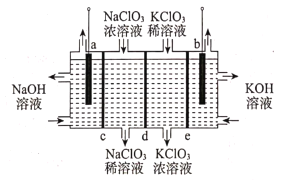
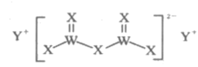
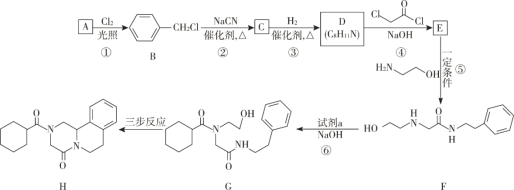
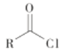
��Ŀ����
����Ŀ�������£���1.00mol��L-1�������20.00mL1.00mol��L-1�İ�ˮ�У���ҺpH���¶��������������ı仯������ͼ��ʾ��
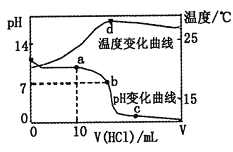
�����й�˵���в���ȷ���ǣ� ��
A. a����Һ������Ũ�ȴ�С��ϵ��c��NH4+����c��Cl-����c��OH-����c��H+��
B. b����Һ������Ũ�ȴ�С��ϵ��c��NH4+��=c��Cl-����c��H+��=c��OH-��
C. c����Һ������Ũ�ȴ�С��ϵ��c��NH4+��+c��H+��=c��Cl-��+c��OH-��
D. d��ʱ��Һ�¶ȴﵽ��ߣ�֮���¶������½���ԭ����NH3��H2O����
���𰸡�D
��������
A.a����Һ��Ϊ�����ʵ���Ũ�ȵ�![]() ��
��![]() ������Һ�Լ��ԣ��ʰ�ˮ�ĵ���̶ȴ���NH4Cl��ˮ��̶���������
������Һ�Լ��ԣ��ʰ�ˮ�ĵ���̶ȴ���NH4Cl��ˮ��̶���������![]() ��A����ȷ��
��A����ȷ��
B.b����Һ�����ԣ���![]() �����ݵ���غ���
�����ݵ���غ���![]() ������
������![]() ��B����ȷ��
��B����ȷ��
C.c����Һ��������NH4Cl��HCl�����ݵ���غ���![]() �� C����ȷ��
�� C����ȷ��
D.d��ʱ���ǡ���кͣ��ų�������࣬��Һ�¶ȴﵽ��ߣ��кͷ�Ӧ��������������¶ȵͣ���������ļ�������Һ�¶���������NH3��H2O�����أ�D�����ѡD��

����Ŀ������5Gʱ���ĵ������뵼����Ͻ�ӭ�����ٷ�չ�����Ȼ�����(POCl3)�������뵼����Ӽ������άԭ�ϡ�һ�о�С����ʵ��������PCl3��SO2��Cl2��60��65��Cʱ��Ӧ�Ʊ�POCl3��SOCl3��ʵ��װ����ͼ��ʾ��������Ʊ�װ��δ��������
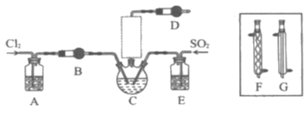
���Ͽ�Ƭ��
���� | �۵�/��C | �е�/��C | ���� |
PCl3 | -93.6 | 76.1 | ��ˮ����ˮ�⣬����O2��Ӧ |
POCl3 | 1.25 | 105.8 | ��ˮ����ˮ�⣬������PCl3 |
SOCl3 | -105 | 78.8 | ��ˮ����ˮ�⣬�����ֽ� |
��1���÷�Ӧ�Ļ�ѧ����ʽΪ___��
��2��A��Bװ���е��Լ��ֱ���___��___��
��3��װ��E��������___��
��4����Ӧװ�õ����߿���δ�������������ѡ��___������F������G��)��������___��
��5����Ӧ�������ᴿPOCl3�IJ�����___����������ƣ���
��6���ⶨij���Ӽ���POCl3�ĺ��������ʲ����뷴Ӧ����ȷ��ȡ4.000g��Ʒ��ˮ��ƿ��ҡ������ȫˮ�⣬��ˮ��Һ���250mL��Һ��ȡ25.00mL����ƿ�У�����0.4000molL-1��AgNO3��Һ25.00mL���ټ�����������������������NH4Fe(SO4)2��ָʾ������0.l000molL-1KSCN����Һ�ζ�������AgNO3���յ㣬����KSCN����Һ22.00mL������֪��Ksp(AgCl)=3.2��10-10��Ksp(AgSCN)=2��10-12��Ag3PO4���������ᣬPOCl3����Է�������Ϊ153.5]
�ټ�����������������Ŀ����___��
��POCl3����������Ϊ___��������һλС����
����Ŀ��ij��ѧ�о���ѧϰС�齫������ط�ĩ��һ�������ۻ�ϸ����������ȣ�����ʵ�����������ò����в�����ˮ�ĺ�ɫ��ĩX����̽����
��̽��Ŀ�ģ�������ɫ��ĩX����ɣ����������ʵ�顣
����������裩�ú�ɫ��ĩ���ܺ������ۡ��������̡������������е�һ�ֻ��֡�
��������֤��������������ɫ��ĩ�����ֱ�������
���������ۣ���ɫ��ĩ��_______����MnO2����_______����Fe��________����Fe3O4(�һ�����������ܡ���һ������)��
�����Լ��飩
ʵ�鲽�� | ʵ����� | ʵ������ |
����һ | ȡ������ɫ��ĩ���Թ��У�������ϡ���ᣬ�� | ��ɫ��ĩ�����ܽ⣬�����ݲ��� |
����� | ������һ��Ӧ���Թ��е����ʹ��ˣ�����Һ�еμӼ���KSCN ��Һ���� | ��Һ���ֺ�ɫ |
������ | ȡ����������������Թ��У�������Ũ���ᣬ���� | ����ȫ���ܽ⣬�л���ɫ������� |
�������в�����������ӷ���ʽΪ___________________________________________��
���������飩
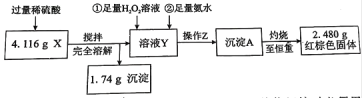
������Z��������__________����ͼ�������У������ճ���ʱ�����õ�����____________(����ĸ)��
����ͬѧ��Ϊ������������������H2O2���������費�䣬ֻҪ�ڿ����г�ַ����ԿɴﵽĿ�ġ�����������(�û�ѧ����ʽ��ʾ)__________________________________________��
��ͨ���������ݣ��ó�4.116 g��ɫ��ĩX�и��ɷֵ����ʵ���Ϊ_________________��