��Ŀ����
2����1�������������ʣ���O2 ��Na2O2 ��Ca��OH��2 ��NH3 ��H2O2 ��CaCl2 ��NH4Cl�������Ӽ��ͷǼ��Լ����ɵ������Ǣڣ�ֻ�ɼ��Լ����ɵ������Ǣܣ��ɼ��Լ��ͷǼ��Լ����ɵ������Ǣݣ������Ӽ��ͼ��Լ����ɵ������Ǣۢߣ����ڹ��ۻ�������Ǣܢݣ���2����ͬ������D2O��H2O��������֮����9��10����ͬ���ʵ�����D2O��H2O��������֮����5��4��
���� ��1��һ����˵�����ý����ͻ��÷ǽ���Ԫ��֮�����γ����Ӽ�����ͬ�ǽ���Ԫ��֮�����γɼ��Լ���ͬ�ַǽ���Ԫ��֮�����γɷǼ��Լ����������Ӽ��Ļ����������ӻ����ֻ�����ۼ��Ļ������ǹ��ۻ�����ݴ˷������
��2���ȼ���D2O��H2O�����ʵ������ټ��������������֮�ȣ�D2O�к���10�����ӣ�H2O����8�����ӣ�
��� �⣺��1����O2��ֻ���Ǽ��Լ���
��Na2O2�������������������֮��������Ӽ�������O-O�Ǽ��Լ����������ӻ����
��Ca��OH��2�к������Ӽ���O-H���Լ����������ӻ����
��NH3��ֻ������H-N���Լ������ڹ��ۻ����
��H2O2 �д���H-O���Լ���O-O�Ǽ��Լ������ڹ��ۻ����
��CaCl2ֻ�������Ӽ����������ӻ����
��NH4Cl�д������Ӽ��ͼ��Լ����������ӻ����
�����Ӽ��ͷǼ��Լ����ɵ������Ǣڣ�ֻ�ɼ��Լ����ɵ������Ǣܣ��ɼ��Լ��ͷǼ��Լ����ɵ������Ǣݣ������Ӽ��ͼ��Լ����ɵ������Ǣۢߣ����ڹ��ۻ��������
�ܢݣ�
�ʴ�Ϊ���ڣ��ܣ��ݣ��ۢߣ��ܢݣ�
��2��������ͬ��D2O��H2O�����ʵ���֮��Ϊ$\frac{1}{20}$��$\frac{1}{18}$=9��10������������֮��Ϊ9��10��D2O�к���10�����ӣ�H2O����8�����ӣ�����ͬ���ʵ�����D2O��H2O��������֮����5��4��
�ʴ�Ϊ��9��10��5��4��
���� ���⿼�������ʺͻ�ѧ���Ĺ�ϵ�����ʵ����ļ��㡢�������������������ļ��㣬��ȷ�����д��ڵĻ�ѧ���Լ�ԭ�ӽṹ�ǽⱾ��ؼ�����Ŀ�ѶȲ���

| A�� | ��������ܶȺ㶨���� | B�� | ����������ɫ���ٸı� | ||
| C�� | ��������ƽ��Ħ�������㶨���� | D�� | H2��I2��HI��Ũ����� |
| A�� | H2O��NH3 | B�� | NaCl��HNO3 | C�� | CaF2��CsCl | D�� | SiCl4��CH4 |
| A�� | �ں����������п�鱣����Dz��ܸ�ʴ�Dz������������������������� | |
| B�� | ����������Ʒ�ĶƲ�����ʱ���Ʋ����ܶ�����Ʒ�𱣻����� | |
| C�� | �����������ڿ�������ѧ��ʴ�����䰵 | |
| D�� | �ɽ��������ֹ������ֱ����Դ�����������Ա��������ܸ�ʴ |
| ���鷽�� | ������ | ��ɫ�� | ���巨 |
| ���� | ��Ӧ���г����������ܽ� | ��Ӧ������ɫ�仯 | ��Ӧ����������� |
| A�� | NH4+�����巨 | B�� | Cl-�������� | C�� | I2����ɫ�� | D�� | SO42-�����巨 |
| A�� | 100mL | B�� | 99mL | C�� | 101mL | D�� | 98mL |
| A�� | Na+�ĵ����Ų�ͼ��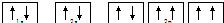 | |
| B�� | HF�ĵ���ʽ��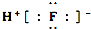 | |
| C�� | Fe2+�ĺ�������Ų�ʽ��1s22s22p63s23p63d6 | |
| D�� | N2�Ľṹ��ʽ����N��N�� |
