��Ŀ����
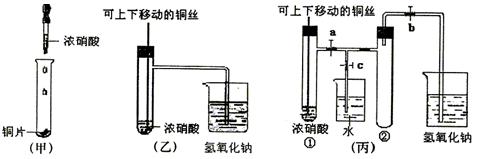
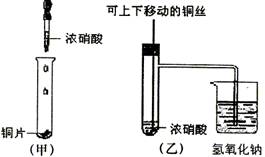
��ͼ���ǿα�����֤ͭ��Ũ���ᷴӦ��װ�ã��ҡ�����ʦ������ʾʵ��Ľ����װ�ã�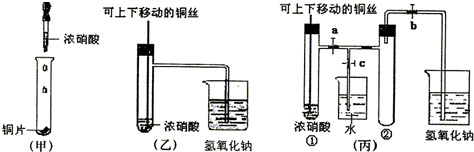
��1��д��ͭ��Ũ���ᷴӦ�����ӷ���ʽ
��2���ͼ�װ����ȣ���װ�õ��ŵ���
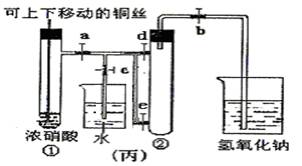
��3��Ϊ�˽�һ����֤NO2��ˮ�ķ�Ӧ��ijѧ������˱�װ�ã���ʵ��ʱ�ȹرյ��ɼ�
��4��������������Թܺ�ͭ˿��������Һ���룬��ʹ�ձ��е�ˮ������Թ�Ӧ��β���
��5�����Թ��е�NO2��ˮ��ַ�Ӧ��������Һ���ʵ���Ũ�ȵ����ֵ��

��1��д��ͭ��Ũ���ᷴӦ�����ӷ���ʽ
Cu+4H++2NO3_=Cu2++2NO2��+2H2O
Cu+4H++2NO3_=Cu2++2NO2��+2H2O
����2���ͼ�װ����ȣ���װ�õ��ŵ���
�ٿ��Կ��Ʒ�Ӧ������NO2���壬��ֹ��Ⱦ������
�ٿ��Կ��Ʒ�Ӧ������NO2���壬��ֹ��Ⱦ������
��3��Ϊ�˽�һ����֤NO2��ˮ�ķ�Ӧ��ijѧ������˱�װ�ã���ʵ��ʱ�ȹرյ��ɼ�
c
c
���ٴ��ɼ�a��b
a��b
������ʹNO2����������Թܣ���4��������������Թܺ�ͭ˿��������Һ���룬��ʹ�ձ��е�ˮ������Թ�Ӧ��β���
�ȹر�b���ٹر�a��Ȼ���c��������ס����ˮ����ë�������ȣ��Թܢ�
�ȹر�b���ٹر�a��Ȼ���c��������ס����ˮ����ë�������ȣ��Թܢ�
����5�����Թ��е�NO2��ˮ��ַ�Ӧ��������Һ���ʵ���Ũ�ȵ����ֵ��
1/22.4mol?L-1��0.045mol?L-1
1/22.4mol?L-1��0.045mol?L-1
�������������״�����㣩����������1������ͭ��Ũ���ᷴӦ��������ͭ������������ˮ��
��2�����ݷ�Ӧ�ɿ��ƣ���ʱ����ֹͣ�����ݷ�Ӧ����Ⱦ��
��3�����ݴ˲�����Ϊ��NO2����������Թܣ�
��4���ö���������ˮ�Ӵ�������Ӧ��
��5������NO2��ˮ��Ӧ�����Թܵ��ݻ�ΪVL��������ʵ����ʵ�������Һ�������
��2�����ݷ�Ӧ�ɿ��ƣ���ʱ����ֹͣ�����ݷ�Ӧ����Ⱦ��
��3�����ݴ˲�����Ϊ��NO2����������Թܣ�
��4���ö���������ˮ�Ӵ�������Ӧ��
��5������NO2��ˮ��Ӧ�����Թܵ��ݻ�ΪVL��������ʵ����ʵ�������Һ�������
����⣺��1����ͭ��Ũ���ᷴӦ��������ͭ������������ˮ���ʴ�Ϊ��Cu+4H++2NO3_=Cu2++2NO2��+2H2O��
��2����װ����ʹ��Ӧ�ɿ��ƣ���ʱ����ֹͣ��ͬʱ����NO2���壬��ֹ��Ⱦ������
�ʴ�Ϊ���ٿ��Կ��Ʒ�Ӧ������NO2���壬��ֹ��Ⱦ������
��3��Ϊ��NO2����������Թܣ�Ӧ�ȹر�c��Ȼ���a��b���ʴ𰸣��ر�c��a��b��
��4��Ϊ���ö���������ˮ�Ӵ���������Ӧ�ȹر�b���ٹر�a��Ȼ���c��������ס����ˮ����ë�������ȣ��Թܢڣ��ʴ�Ϊ���ȹر�b���ٹر�a��Ȼ���c��������ס����ˮ����ë�������ȣ��Թܢڣ������𰸾����֣���
��5�����Թܵ��ݻ�ΪVL������NO2��ˮ��Ӧ��3NO2 +H2O=2HNO3 +NO
��
��Ӧ����Һ�е�����HNO3�����ʵ���Ϊ
��
mol����Һ�����ΪV��
L��
������Һ���ʵ���Ũ��Ϊ��
mol?L-1=0.045mol?L-1��
�ʴ�Ϊ��0.045mol?L-1��
��2����װ����ʹ��Ӧ�ɿ��ƣ���ʱ����ֹͣ��ͬʱ����NO2���壬��ֹ��Ⱦ������
�ʴ�Ϊ���ٿ��Կ��Ʒ�Ӧ������NO2���壬��ֹ��Ⱦ������
��3��Ϊ��NO2����������Թܣ�Ӧ�ȹر�c��Ȼ���a��b���ʴ𰸣��ر�c��a��b��
��4��Ϊ���ö���������ˮ�Ӵ���������Ӧ�ȹر�b���ٹر�a��Ȼ���c��������ס����ˮ����ë�������ȣ��Թܢڣ��ʴ�Ϊ���ȹر�b���ٹر�a��Ȼ���c��������ס����ˮ����ë�������ȣ��Թܢڣ������𰸾����֣���
��5�����Թܵ��ݻ�ΪVL������NO2��ˮ��Ӧ��3NO2 +H2O=2HNO3 +NO
| V |
| 22.4 |
| V |
| 22.4 |
| 2 |
| 3 |
��Ӧ����Һ�е�����HNO3�����ʵ���Ϊ
| V |
| 22.4 |
| 2 |
| 3 |
| 2 |
| 3 |
������Һ���ʵ���Ũ��Ϊ��
| 1 |
| 22.4 |
�ʴ�Ϊ��0.045mol?L-1��
������������Ҫ������ͭ��Ũ����ķ�Ӧ��ͬʱ��������ҺŨ�ȵļ��㣬������ѧ������������

��ϰ��ϵ�д�
�����Ŀ