��Ŀ����
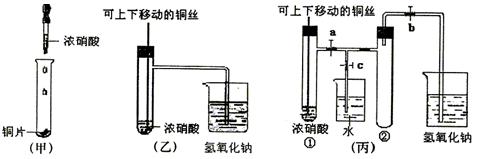
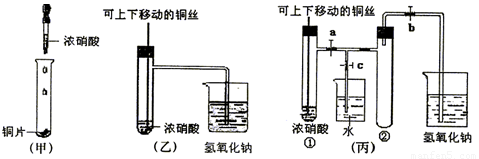
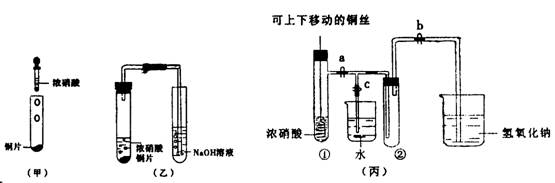
(10��) ��ͼ���ǿα�����֤ͭ��Ũ���ᷴӦ��װ�ã��ҡ�����ʦ������ʾʵ��Ľ����װ�ã�
��1�� д��ͭ��Ũ���ᷴӦ�����ӷ���ʽ ��
��2���ͼ�װ����ȣ���װ�õ��ŵ���
��3��Ϊ�˽�һ����֤NO2��ˮ�ķ�Ӧ��ijѧ������˱�װ��,��ʵ��ʱ�ȹرյ��ɼ�
���ٴ��ɼ� ������ʹNO2����������Թܡ�
��4��������������Թܺ�ͭ˿��������Һ���룬��ʹ�ձ��е�ˮ������Թ�Ӧ��β�
�� ��
��5�����Թ��е�NO2��ˮ��ַ�Ӧ��������Һ���ʵ���Ũ�ȵ����ֵ��
�������������״�����㣩��
��1��Cu + 4H+ + 2NO3_= Cu2+ + 2NO2��+ 2H2O ��2�֣�
��2���ٿ��Կ��Ʒ�Ӧ����1�֣�������NO2���壬��ֹ��Ⱦ��������1�֣�
��3���ر�c����a��b����2�֣�
��4���ȹر�b���ٹر�a, Ȼ���c��������ס����ˮ����ë�������ȣ��Թܢڡ��������𰸾����֣� ��2�֣�
��5��1/22.4mol��L��1��0.045mol��L��1 ��2�֣�
����:

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�