��Ŀ����
(10��)��ͼ���ǿα�����֤ͭ��Ũ���ᷴӦ��װ�ã��ҡ�����ʦ������ʾʵ��Ľ����װ�ã�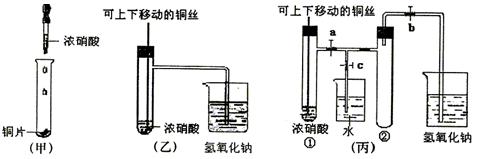
��1�� д��ͭ��Ũ���ᷴӦ�����ӷ���ʽ ��
��2���ͼ�װ����ȣ���װ�õ��ŵ���
��3��Ϊ�˽�һ����֤NO2��ˮ�ķ�Ӧ��ijѧ������˱�װ��,��ʵ��ʱ�ȹرյ��ɼ�
���ٴ��ɼ� ������ʹNO2����������Թܡ�


��4��������������Թܺ�ͭ˿��������Һ���룬��ʹ�ձ��е�ˮ������Թ�Ӧ��β�
�� ��
��5�����Թ��е�NO2��ˮ��ַ�Ӧ��������Һ���ʵ���Ũ�ȵ����ֵ��
�������������״�����㣩��
��1��Cu + 4H+ + 2NO3_ = Cu2+ + 2NO2��+ 2H2O ��2�֣�
��2���ٿ��Կ��Ʒ�Ӧ����1�֣�������NO2���壬��ֹ��Ⱦ��������1�֣�
��3���ر�c����a��b����2�֣�
��4���ȹر�b���ٹر�a, Ȼ���c��������ס����ˮ����ë�������ȣ��Թܢڡ��������𰸾����֣� ��2�֣�
��5��1/22.4mol��L��1��0.045mol��L��1 ��2�֣�
����

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д���A������ͼ��ʾ�����ס�������װ�в�ͬ���ʵ���Ͳ�õ�������������������Ͳ�ڵ�����ѹ������Ͳ�ڣ������±����еIJ�ͬʵ�飨������ͬ��ͬѹ�²ⶨ����
![]()
ʵ����� | ����Ͳ������ | ����Ͳ������ | ����Ͳ������ |
1 | 10 mL FeSO4��Һ | 10 mL NH3 | ���ɰ�ɫ���������ɫ |
2 | 20 mL H2S | 10 mL SO2 |
|
3 | 30 mL NO2����Ҫ�� | 10 mL H2O(l) | ʣ����ɫ���壬�����Զ�����ѹ�� |
4 | 15 mL Cl2 | 40 mL NH3 |
|
�Իش��������⣺
��1��ʵ��1�У��������ձ�Ϊ___________ɫ��д��������ɫ�Ļ�ѧ����ʽ_______________��
��2��ʵ��2����Ͳ�ڵ������ǣ���________���ɣ�����___________�ƶ�������⡱�����ڡ�����������Ӧ�����Ͳ���������IJ������壬��ȷ�Ĵ��������ǽ���ͨ��__________��Һ�С�
��3��ʵ��3�У����е�30 mL������NO2��N2O4�Ļ�����壬��ô�������ʣ�����ɫ������__________��д��NO2��H2O��Ӧ�Ļ�ѧ����ʽ_______________________________��
��4��ʵ��4�У���֪��3Cl2+2NH3![]() N2+6HCl������Ͳ���������ƶ�����Ͳ���а��̲����⣬�������ɫ�仯Ϊ___________�������Ͳ��ʣ����������ԼΪ______________mL��
N2+6HCl������Ͳ���������ƶ�����Ͳ���а��̲����⣬�������ɫ�仯Ϊ___________�������Ͳ��ʣ����������ԼΪ______________mL��
��B��ijʵ��С��������װ�ý����Ҵ���������ʵ�顣

��1��ʵ�������ͭ�����ֺ�ɫ�ͺ�ɫ�����������д����Ӧ�Ļ�ѧ��Ӧ����ʽ
_____________________________________________________________________
_____________________________________________________________________��
�ڲ��Ϲ������������£�Ϩ��ƾ��ƣ���Ӧ���ܼ������У�˵�����Ҵ�������Ӧ��________��Ӧ��
��2����������ˮԡ���ò���ͬ��
��������____________________���ҵ�������_____________________��
��3����Ӧ����һ��ʱ������Թ�a�����ռ�����ͬ�����ʣ�������____________________________������ƿ���ռ������������Ҫ�ɷ���______________��
��4�����Թ�a���ռ�����Һ������ɫʯ����ֽ���飬��ֽ�Ժ�ɫ��˵��Һ���л�����__________��Ҫ��ȥ�����ʣ������ڻ��Һ�м���______________����д��ĸ����
a.�Ȼ�����Һ b.��
c.̼��������Һ d.���Ȼ�̼
Ȼ����ͨ��_____________����ʵ��������ƣ����ɳ�ȥ��