��Ŀ����
����������Ч����SO2�Կ�������Ⱦ��
(1)��ú�м���ʯ��ʯ�ɼ���ȼ�ղ�����SO2�ĺ������÷�Ӧ�Ļ�ѧ����ʽ��
_______________________________��
(2)��ˮ�������ԣ���Ҫ����Na����K����Ca2����Mg2����Cl����SO42����Br����HCO3���ȡ���SO2�����������ú�ˮ�����乤��������ͼ��ʾ��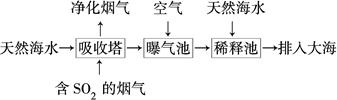
������������ͨ�������Ŀ����_____________________________________��
��ͨ��������������к�ˮ����Ȼ��ˮ��ȣ�Ũ�������Բ�ͬ��������________��
a��Cl�������� b��SO42�������� c��Br�������� d��HCO3��
(3)��NaOH��Һ���������е�SO2�������õ�Na2SO3��Һ���е�⣬�ɵõ�NaOH��ͬʱ�õ�H2SO4����ԭ����ͼ��ʾ(�缫����Ϊʯī)��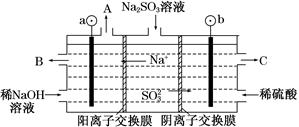
��ͼ��a��Ҫ���ӵ�Դ��________(���������)����C��������������________��
��SO32���ŵ�ĵ缫��ӦʽΪ____________________________��
�۵�����������������������ǿ����ƽ���ƶ���ԭ������ԭ��
__________________________________________��
(1)2SO2��O2��2CaCO3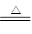 2CaSO4��2CO2
2CaSO4��2CO2
(2)�ٽ�H2SO3��HSO3��������ΪSO42������bd
(3)�ٸ�������
��SO32����2e����H2O=SO42����2H��
��H2O H����OH����������H���ŵ�����H2��c(H��)��С��ˮ�ĵ���ƽ�������ƶ���������ǿ
H����OH����������H���ŵ�����H2��c(H��)��С��ˮ�ĵ���ƽ�������ƶ���������ǿ
����

 ����ѵ�����⿼ϵ�д�
����ѵ�����⿼ϵ�д���.�ס���Ԫ�صĵ��ʺͻ�����Ӧ�ù㷺��
��1����Ԫ�ص�ԭ�ӽṹʾ��ͼ�� ��
��2��������뽹̿��ʯӢɰ���,�ڵ�¯�м��ȵ�1 500 �����ɰ���,��ӦΪ:
2Ca3��PO4��2+6SiO2 6CaSiO3+P4O10
6CaSiO3+P4O10
10C+P4O10 P4+10CO
P4+10CO
ÿ����1 mol P4ʱ,���� mol���ӷ���ת�ơ�
��3����������ƣ�Na2S2O3���dz��õĻ�ԭ������ά����C����ѧʽC6H8O6����ˮ��Һ�м������I2��Һ,ʹά����C��ȫ����,ʣ���I2��Na2S2O3��Һ�ζ�,�ɲⶨ��Һ��ά����C�ĺ����������ķ�ӦΪ:
C6H8O6+I2 C6H6O6+2H++2I-
C6H6O6+2H++2I-
2S2 +I2
+I2 S4
S4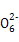 +2I-
+2I-
��һ�������ijά����C��Һ�м���a mol/L I2��ҺV1 mL,��ַ�Ӧ��,��Na2S2O3��Һ�ζ�ʣ���I2,����b mol/L Na2S2O3��ҺV2 mL������Һ��ά����C�����ʵ����� mol��
��4����������Һ��,����أ�KIO3�����������ƿɷ������·�Ӧ:
2I +5S
+5S +2H+
+2H+ I2+5S
I2+5S +H2O
+H2O
���ɵĵ�����õ�����Һ����,���ݷ�Ӧ��Һ������ɫ�����ʱ���������÷�Ӧ�����ʡ�
ijͬѧ���ʵ�����±���ʾ:
| | 0.01 mol/LKIO3������Һ�������ۣ������/mL | 0.01 mol/LNa2SO3��Һ�����/mL | H2O�����/mL | ʵ���¶�/�� | ��Һ������ɫʱ����ʱ��/s |
| ʵ��1 | 5 | V1 | 35 | 25 | |
| ʵ��2 | 5 | 5 | 40 | 25 | |
| ʵ��3 | 5 | 5 | V2 | 0 | |
��ʵ���Ŀ���� ;����V2= mL��
��.ϡ��Ԫ���DZ����ս����Դ,�ҹ����̲�����������λ��
��5���棨Ce���ǵؿ��к�����ߵ�ϡ��Ԫ�ء��ڼ���������CeCl3����ˮ��,��ˮCeCl3���ü���CeCl3��6H2O��NH4Cl��������ķ������Ʊ�������,NH4Cl�������� ��
��6����ijǿ���Ի��ϡ����Һ�м���H2O2,����pH��3,Ce3+ͨ�����з�Ӧ�γ�Ce��OH��4�������Է��롣��ɷ�Ӧ�����ӷ���ʽ:
Ce3++H2O2+H2O
 Ce��OH��4��+ ��
Ce��OH��4��+ �� 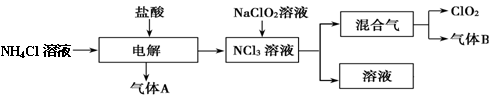
 Cu2++Cu������������Ϣ,����Լ������յĻ�ѧ֪ʶ,�ش�:
Cu2++Cu������������Ϣ,����Լ������յĻ�ѧ֪ʶ,�ش�:

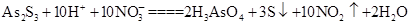 ��������2 mol H3AsO4����Ӧ��ת�Ƶ��ӵ����ʵ���Ϊ______�������÷�Ӧ��Ƴ�һԭ��أ���NO2Ӧ����______����������������������ݳ���
��������2 mol H3AsO4����Ӧ��ת�Ƶ��ӵ����ʵ���Ϊ______�������÷�Ӧ��Ƴ�һԭ��أ���NO2Ӧ����______����������������������ݳ��� Al(OH)3��N2��NaAlO2
Al(OH)3��N2��NaAlO2