��Ŀ����
����ͼ��ʯī���缫�ĵ����У�����500 mL��һ�����ʵ�ij��ɫ��Һ���е�⣬�۲쵽A�缫�����к�ɫ�Ĺ�̬�������ɣ�B�缫����ɫ�������ɣ�����Һ�е�ԭ��������ȫ����ֹͣ��⣬ȡ��A�缫��ϴ�ӡ�����������缫����1.6 g����ش��������⣺

(1)B�缫������Ӧ�ĵ缫��Ӧʽ_________________________��
(2)д�����ʱ��Ӧ�����ӷ���ʽ_________________________________________��
(3)������Һ��H+�����ʵ���Ũ��Ϊ________��Ҫʹ������Һ�ָ������ǰ��״̬���������________��������Ϊ________g��(������ǰ����Һ���������)
(4)ԭ��Һ�п��ܺ��е��������Ϊ�� ��
A CO32- B Cl- C SO42-
���ʵ������������ӣ�д���������裬ʵ�������ʵ�����________________________
�� 4OH-��4e-=2H2O+O2��
�� 2Cu2++2H2O 2Cu+O2��+4H+
��3��0.1mol��L-1 CuO 2
2Cu+O2��+4H+
��3��0.1mol��L-1 CuO 2
(4)C ȡ������Һ���Թ��У�������������������ɣ��ټ��Ȼ�����Һ�����ɰ�ɫ������֤����Һ����SO42-
������������绯ѧ��Ӧ�á�
��1��A�缫�����к�ɫ�Ĺ�̬�������ɣ�B�缫����ɫ�������ɣ�˵��A�缫���������γɵĺ�ɫ��̬������ͭ��B�缫����������������ɫ�������������缫��ӦʽΪ4OH-��4e-=2H2O+O2����
��2�������ܷ�ӦʽΪ2Cu2++2H2O 2Cu+O2��+4H+��
2Cu+O2��+4H+��
��3�����ݷ�Ӧʽ��֪��������ͭ��1.6g�����ʵ�����0.025mol�����Ե��������ӵ����ʵ�����0.05mol����Ũ��Ϊ0.05mol��0.5L��0.1mol/L�����ڴ���Һ�г�������ͭ��������������Ҫ��������ͭ����ʹ��Һ�ָ������ǰ��״̬����������0.025mol��80g/mol��2.0g��
��4������������OH���ŵ磬�������Ҳ�Ǻ����������Ϊ̼��ͭ������ˮ�����Դ���C������SO42-���������ữ���Ȼ�����Һ��

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д� ����ͼ��ʯī���缫�ĵ����У�����500mL��һ�����ʵ�ij��ɫ��Һ���е�⣬�۲쵽A�缫�����к�ɫ�Ĺ�̬�������ɣ�B�缫����ɫ�������ɣ�����Һ�е�ԭ��������ȫ����ֹͣ��⣬ȡ��A�缫��ϴ�ӡ�����������缫����1.6g����ش��������⣺
����ͼ��ʯī���缫�ĵ����У�����500mL��һ�����ʵ�ij��ɫ��Һ���е�⣬�۲쵽A�缫�����к�ɫ�Ĺ�̬�������ɣ�B�缫����ɫ�������ɣ�����Һ�е�ԭ��������ȫ����ֹͣ��⣬ȡ��A�缫��ϴ�ӡ�����������缫����1.6g����ش��������⣺ ����ͼ��ʯī���缫�ĵ����У�����500mL��һ�����ʵ�ij��ɫ��������Һ���е�⣬�۲쵽A�缫�����к�ɫ�Ĺ�̬�������ɣ�B�缫����ɫ�������ɣ���ش��������⣺
����ͼ��ʯī���缫�ĵ����У�����500mL��һ�����ʵ�ij��ɫ��������Һ���е�⣬�۲쵽A�缫�����к�ɫ�Ĺ�̬�������ɣ�B�缫����ɫ�������ɣ���ش��������⣺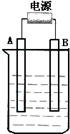 ����ͼ��ʯī���缫�ĵ����У�����500mL��һ�����ʵ�ij��ɫ��Һ���е�⣬�۲쵽A�缫�����к�ɫ�Ĺ�̬�������ɣ�B�缫����ɫ�������ɣ���ش��������⣺
����ͼ��ʯī���缫�ĵ����У�����500mL��һ�����ʵ�ij��ɫ��Һ���е�⣬�۲쵽A�缫�����к�ɫ�Ĺ�̬�������ɣ�B�缫����ɫ�������ɣ���ش��������⣺