��Ŀ����
 ����ͼ��ʯī���缫�ĵ����У�����500mL��һ�����ʵ�ij��ɫ��Һ���е�⣬�۲쵽A�缫�����к�ɫ�Ĺ�̬�������ɣ�B�缫����ɫ�������ɣ���ش��������⣺
����ͼ��ʯī���缫�ĵ����У�����500mL��һ�����ʵ�ij��ɫ��Һ���е�⣬�۲쵽A�缫�����к�ɫ�Ĺ�̬�������ɣ�B�缫����ɫ�������ɣ���ش��������⣺��1����д��B��������Ƽ���Ӧʽ��
��2��д�����ʱ��Ӧ�������ӷ���ʽ
��3��������Һ�е�ԭ��������ȫ����ֹͣ��⣬ȡ��A�缫��ϴ�ӡ�����������缫����1.6g��������Һ��pHΪ
��4����ԭ��ҺΪ1L K2SO4��CuSO4�Ļ����Һ����c��SO42-��=2.0mol/L����ͼװ�õ�⣬���������ռ���22.4L���壨��״����ʱ��ֹͣ��⣮��ԭ��Һ�е�c��K+��Ϊ���٣������ʾ������̣�
������������Һʱ��A�����н�������ͭ���ɣ���A��������B������������������ɫ�������ɣ��������������������ҺΪ�������ͭ�Σ�
��1��B�����������������������ӷŵ�����������
��2�����������������ӷŵ�����������������ͭ���ӷŵ�����ͭ��
��3������ͭ��������֮��Ĺ�ϵʽ����������Ũ�ȣ�pH=-lgc(H+)�����ݡ�����ʲô����ʲô����ԭ��������ʣ�
��4���������Һʱ�����������������ӷŵ磬��������ͭ���ӷŵ�������ӷŵ磬����ת�Ƶ����غ��������ͭ�����ʵ������ٸ��ݵ������Һ�е���غ���������Ũ�ȣ�
��1��B�����������������������ӷŵ�����������
��2�����������������ӷŵ�����������������ͭ���ӷŵ�����ͭ��
��3������ͭ��������֮��Ĺ�ϵʽ����������Ũ�ȣ�pH=-lgc(H+)�����ݡ�����ʲô����ʲô����ԭ��������ʣ�
��4���������Һʱ�����������������ӷŵ磬��������ͭ���ӷŵ�������ӷŵ磬����ת�Ƶ����غ��������ͭ�����ʵ������ٸ��ݵ������Һ�е���غ���������Ũ�ȣ�
����⣺��1��A�缫�����к�ɫ�Ĺ�̬�������ɣ�B�缫����ɫ�������ɣ�˵��A�缫��������ͭ����AΪ������BΪ���������������������ӷŵ������������缫��ӦʽΪ4OH--4e-=2H2O+O2����
�ʴ�Ϊ��������4OH--4e-=2H2O+O2����
��2��A�缫�����к�ɫ�Ĺ�̬�������ɣ�B�缫����ɫ�������ɣ�˵��A�缫��������ͭ����AΪ������BΪ���������������������ӷŵ��������������Ե�ط�ӦʽΪ��2Cu2++2H2O
2Cu+O2��+4H+��
�ʴ�Ϊ��2Cu2++2H2O
2Cu+O2��+4H+��
��3������ͭ�����ʵ���=
=0.025mol������2Cu2++2H2O
2Cu+O2��+4 H+��ͭ�������ӵĹ�ϵʽ��n��H+��=2n��Cu��=0.05mol��c�� H+��=
=0.1mol/L������Һ��pH=1��
��������������������������ͭ���������������ʾ��൱��CuO������ͭԭ���غ��m��CuO��=n��CuO����M��CuO��=n��Cu����M��CuO��=0.025mol��80g/mol=2g��
��������ʲô����ʲô��ԭ��֪��Ϊ��ʹ��Һ�ָ�ԭ״�����Լ���2g����ͭ��Ҳ���Լ���̼��ͭ������ԭ���غ��m��CuCO3��=n��CuCO3����M��CuCO3��=n��CuO��M��CuCO3��=0.025mol��124g/mol=3.2g��
�ʴ�Ϊ��1��CuO 2����CuCO33.2��
��4���������Һʱ�����������������ӷŵ磬��������ͭ���ӷŵ�������ӷŵ磬�ɵ����غ��֪��2n��Cu2+��+2n��H2��=4n��O2����2n��Cu2+��+2��
=4��
��
��n��Cu2+��=1mol��
�ɵ���غ��֪��c��K+��+2c��Cu2+��=2c��SO42-����c��K+��+2��
=2��2.0mol/L��
c��K+��=2mol/L��
��ԭ��Һ��c��K+��Ϊ2mol/L��
�ʴ�Ϊ��������4OH--4e-=2H2O+O2����
��2��A�缫�����к�ɫ�Ĺ�̬�������ɣ�B�缫����ɫ�������ɣ�˵��A�缫��������ͭ����AΪ������BΪ���������������������ӷŵ��������������Ե�ط�ӦʽΪ��2Cu2++2H2O
| ||
�ʴ�Ϊ��2Cu2++2H2O
| ||
��3������ͭ�����ʵ���=
| 1.6g |
| 64g/mol |
| ||
| 0.05mol |
| 0.5L |
��������������������������ͭ���������������ʾ��൱��CuO������ͭԭ���غ��m��CuO��=n��CuO����M��CuO��=n��Cu����M��CuO��=0.025mol��80g/mol=2g��
��������ʲô����ʲô��ԭ��֪��Ϊ��ʹ��Һ�ָ�ԭ״�����Լ���2g����ͭ��Ҳ���Լ���̼��ͭ������ԭ���غ��m��CuCO3��=n��CuCO3����M��CuCO3��=n��CuO��M��CuCO3��=0.025mol��124g/mol=3.2g��
�ʴ�Ϊ��1��CuO 2����CuCO33.2��
��4���������Һʱ�����������������ӷŵ磬��������ͭ���ӷŵ�������ӷŵ磬�ɵ����غ��֪��2n��Cu2+��+2n��H2��=4n��O2����2n��Cu2+��+2��
| 22.4L |
| 22.4L/mol |
| 22.4L |
| 22.4L/mol |
��n��Cu2+��=1mol��
�ɵ���غ��֪��c��K+��+2c��Cu2+��=2c��SO42-����c��K+��+2��
| 1mol |
| 1L |
c��K+��=2mol/L��
��ԭ��Һ��c��K+��Ϊ2mol/L��
���������⿼���˵��ԭ�������ݵ缫����������ȷ�����������ٸ���ͭ�������ӡ�����֮��Ĺ�ϵʽ���м��㣬ע������ԭ���غ㡢����غ�����������Ѷ��еȣ�

��ϰ��ϵ�д�
 ��һ����ͬ���ɽ�����ϵ�д�
��һ����ͬ���ɽ�����ϵ�д� ������Ӧ���ϵ�д�
������Ӧ���ϵ�д�
�����Ŀ
 ����ͼ��ʯī���缫�ĵ����У�����500mL��һ�����ʵ�ij��ɫ��Һ���е�⣬�۲쵽A�缫�����к�ɫ�Ĺ�̬�������ɣ�B�缫����ɫ�������ɣ�����Һ�е�ԭ��������ȫ����ֹͣ��⣬ȡ��A�缫��ϴ�ӡ�����������缫����1.6g����ش��������⣺
����ͼ��ʯī���缫�ĵ����У�����500mL��һ�����ʵ�ij��ɫ��Һ���е�⣬�۲쵽A�缫�����к�ɫ�Ĺ�̬�������ɣ�B�缫����ɫ�������ɣ�����Һ�е�ԭ��������ȫ����ֹͣ��⣬ȡ��A�缫��ϴ�ӡ�����������缫����1.6g����ش��������⣺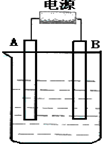 ����ͼ��ʯī���缫�ĵ����У�����500mL��һ�����ʵ�ij��ɫ��������Һ���е�⣬�۲쵽A�缫�����к�ɫ�Ĺ�̬�������ɣ�B�缫����ɫ�������ɣ���ش��������⣺
����ͼ��ʯī���缫�ĵ����У�����500mL��һ�����ʵ�ij��ɫ��������Һ���е�⣬�۲쵽A�缫�����к�ɫ�Ĺ�̬�������ɣ�B�缫����ɫ�������ɣ���ش��������⣺