��Ŀ����
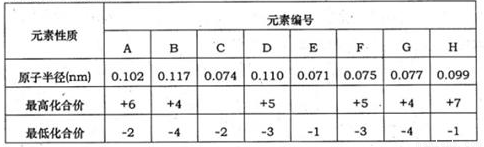
�±�Ϊ���ֶ�����Ԫ�ص�ԭ�Ӱ뾶����Ҫ���ϼۣ����ݱ�����Ϣ�жϣ�����������ȷ����
| Ԫ�ش��� | X | Y | Z | M | N |
| ԭ�Ӱ뾶/nm | 0.160 | 0.143 | 0.102 | 0.071 | 0.099 |
| ��Ҫ���ϼ� | +2 | +3 | +6��-2 | -1 | -1 |
- A.X��Y����������Ӧˮ����ļ��ԣ�Y��X
- B.����������Ӧ��ˮ��������ԣ� H2ZO4��HNO4
- C.��̬�⻯����ȶ��ԣ�HM��H2Z��
- D.M��N�γɵļ����ӵĻ�ԭ�ԣ�N-��M-
D
�������������Ԫ�ص���Ҫ���ϼ��Լ�ԭ�Ӱ뾶��֪��X��Mg��Y��Al��Z��S��M��F��N��Cl��A����ȷ��X��Y����������Ӧˮ����ļ�����X��Y������������Ӧ��ˮ�����������H2ZO4��HNO4��B����ȷ����̬�⻯����ȶ�����HM��H2Z��C����ȷ���ǽ�����Խǿ����Ӧ�����ӵĻ�ԭ��Խǿ����ѡ��D��ȷ����ѡD��
���㣺����Ԫ�������ɵ�Ӧ��
�������������е��Ѷȵ����⣬���������߿������ض�ѧ�������������ͽ��ⷽ����ָ����ѵ����ּ������ѧ��������û���֪ʶ���ʵ�����������������Ĺؼ�����ȷԪ�������ɵĵݱ���ɣ�Ȼ��������������ü��ɣ�����������ѧ����������������
�������������Ԫ�ص���Ҫ���ϼ��Լ�ԭ�Ӱ뾶��֪��X��Mg��Y��Al��Z��S��M��F��N��Cl��A����ȷ��X��Y����������Ӧˮ����ļ�����X��Y������������Ӧ��ˮ�����������H2ZO4��HNO4��B����ȷ����̬�⻯����ȶ�����HM��H2Z��C����ȷ���ǽ�����Խǿ����Ӧ�����ӵĻ�ԭ��Խǿ����ѡ��D��ȷ����ѡD��
���㣺����Ԫ�������ɵ�Ӧ��
�������������е��Ѷȵ����⣬���������߿������ض�ѧ�������������ͽ��ⷽ����ָ����ѵ����ּ������ѧ��������û���֪ʶ���ʵ�����������������Ĺؼ�����ȷԪ�������ɵĵݱ���ɣ�Ȼ��������������ü��ɣ�����������ѧ����������������

��ϰ��ϵ�д�
�����Ŀ


 �±�Ϊ���ֶ�����Ԫ�ػ��ϼۼ�����Ӧԭ�Ӱ뾶�����ݣ���ش��������⣺
�±�Ϊ���ֶ�����Ԫ�ػ��ϼۼ�����Ӧԭ�Ӱ뾶�����ݣ���ش��������⣺