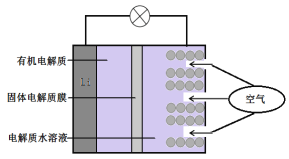
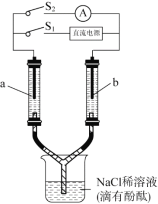
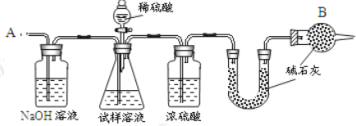
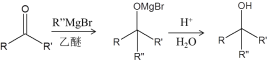��Ŀ����
����Ŀ��A��B��C��D�����ֶ�����Ԫ�أ�E�ǹ���Ԫ�ء�A��B��Cͬ���ڣ�C��Dͬ���壬A��ԭ�ӽṹʾ��ͼΪ ��B��ͬ���ڵ�һ��������С��Ԫ�أ�C�������������δ�ɶԵ��ӣ�E����Χ�����Ų�ʽΪ3d64s2���ش��������⣺
��B��ͬ���ڵ�һ��������С��Ԫ�أ�C�������������δ�ɶԵ��ӣ�E����Χ�����Ų�ʽΪ3d64s2���ش��������⣺
��1��д������Ԫ�صķ��ţ�A________��B________��C________��D________��
��2���û�ѧʽ��ʾ��������Ԫ��������������Ӧˮ����������ǿ����________��������ǿ����__________��
��3����Ԫ�ط��ű�ʾD�������ڵ�һ����������Ԫ����________���縺�ԣ���ϡ�������⣩����Ԫ����_________��
��4��EԪ��ԭ�ӵĺ˵������_________��EԪ�������ڱ��ĵ�_______���ڵ�_______�壬��________����
��5��д��DԪ��ԭ�ӹ��ɵ��ʵĵ���ʽ___________���÷�������_______���Ҽ���_______���м���
���𰸡�Si Na P N HNO3 NaOH Ne F 26 4 VIII d ![]() 1 2
1 2
��������
A��B��C��D�����ֶ�����Ԫ�أ�E�ǹ���Ԫ�ء�A��B��Cͬ���ڣ�C��Dͬ���壬A��ԭ�ӽṹʾ��ͼΪ ����x=2��AΪ14��Ԫ��Si��B��ͬ���ڵ�һ��������С��Ԫ�أ���BΪNaԪ�أ�C�������������δ�ɶԵ��ӣ���CΪPԪ�ء�DΪNԪ�أ�E����Χ�����Ų�ʽΪ3d64s2����EΪ26��Ԫ��Fe��
����x=2��AΪ14��Ԫ��Si��B��ͬ���ڵ�һ��������С��Ԫ�أ���BΪNaԪ�أ�C�������������δ�ɶԵ��ӣ���CΪPԪ�ء�DΪNԪ�أ�E����Χ�����Ų�ʽΪ3d64s2����EΪ26��Ԫ��Fe��
��1��A��B��C��DԪ�صķ��ŷֱ���Si��Na��P��N��
��2����������Ԫ���У��ǽ�������ǿ����NԪ�أ���������ǿ����NaԪ�أ���ˣ�����������Ӧˮ����������ǿ����HNO3��������ǿ����NaOH��
��3��N�������ڼ���2���ڣ���һ����������Ԫ���������ﵽ�ȶ��ṹ��ϡ������Ne���縺�ԣ���ϡ�������⣩����Ԫ����F��
��4��EԪ����Fe��ԭ�ӵĺ˵������26��EԪ�������ڱ��ĵ�4���ڵ�VIII�壬��������Χ�����Ų���֪����Ԫ�����ڱ��е�d����
��5��DԪ��ԭ�ӹ��ɵ���ΪN2�������ʽΪ![]() ���÷�������1��������2��������
���÷�������1��������2��������