��Ŀ����
��1���ϳɰ���ҵ������������Ȼ����ˮ��Ӧ�Ʊ�������Ҫ��ӦΪ��
CH4(g)+ 2H2O(g) 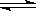 CO2(g)+4H2(g)
CO2(g)+4H2(g)
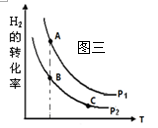
��Ӧ�����������仯��ͼ��ʾ��
��÷�ӦΪ ��Ӧ������ȡ����ȡ���
����֪���ƻ�1mol��ѧ����Ҫ���յ��������±���ʾ��
| ��ѧ�� | C��H | O��H | C=O | H��H |
| ����������kJ/mol�� | a | b | c | d |
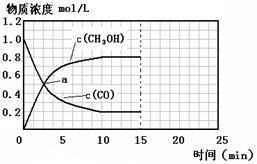
��2��ij�¶��£�10L�ܱ������г���2mol CH4��3mol H2O(g)������
CH4(g)+ 2H2O(g)
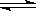 CO2(g)+4H2 (g)��Ӧ�� ��Ӧ���е�4sʱCO2��Ũ��Ϊ0.08mol/L���ٹ�һ��ʱ�䷴Ӧ��ƽ�⣬ƽ��ʱ������ѹǿ����ʼʱ��1.4����
CO2(g)+4H2 (g)��Ӧ�� ��Ӧ���е�4sʱCO2��Ũ��Ϊ0.08mol/L���ٹ�һ��ʱ�䷴Ӧ��ƽ�⣬ƽ��ʱ������ѹǿ����ʼʱ��1.4������ �� ǰ4s��H2O(g)Ũ�ȱ仯��ʾ��ƽ����Ӧ����Ϊ���٣���4sʱ�����������H2���������Ϊ���٣��� ƽ��ʱ��CH4��Ũ���Ƕ��٣�
��Ҫ��д��������̣�
��1�����ȣ�4a+4b-2c-4d
��2��
��
��


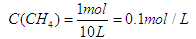
���������������1��������ת����ϵͼ��֪��Ӧ����������������Ũ�ȣ��ʸ÷�ӦΪ���ȷ�Ӧ�����ڷ�ӦCH4(g)+ 2H2O (g)  CO2(g)+4H2(g)���ƻ���Ӧ���л�ѧ������������Ϊ4a+4b���γ��������л�ѧ�����ͷ�����Ϊ2c+4d����÷�Ӧ���յ�����Ϊ4a+4b-2c-4d����2����������ʽ���м��㡣
CO2(g)+4H2(g)���ƻ���Ӧ���л�ѧ������������Ϊ4a+4b���γ��������л�ѧ�����ͷ�����Ϊ2c+4d����÷�Ӧ���յ�����Ϊ4a+4b-2c-4d����2����������ʽ���м��㡣
���㣺���黯ѧ��Ӧ����������ѧ��Ӧ���ʺͻ�ѧƽ��ļ�������֪ʶ��

36g̼����ȫȼ�����������У�COռ����֮һ�����CO2ռ����֮���������֪��2C(s)+O2(g)=2CO(g)��H=-110.5kJ/mol,2CO(g)+O2(g) =2CO2(g)��H=-566kJ/mol,����Щ̼��ȫȼ����ȣ���ʧ�������ǣ� ��
| A��172.5kJ | B��1149kJ | C��517.5kJ | D��283kJ |
��I�����������ѳ�Ϊ��ǰ��δ����һ��ȫ���Կ��⡣Ϊ���Ŀǰȼ��ʹ�ù����еĻ�����Ⱦ���⣬��������ԴΣ�����е�ר���������̫���ܴ�ʹȼ��ѭ��ʹ�õĹ��룬��ͼ��ʾ��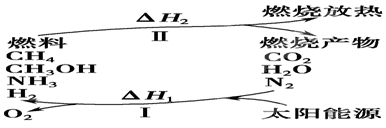
��ش��������⣺
(1)���̢������ת����ʽΪ________��ת��Ϊ________�ܡ�
(2)����ת�������У���H1�ͦ�H2�Ĺ�ϵ��________��
(3)����1 mol��ѧ��������������±���
| ���ۼ� | H��N | H��O | N��N | O��O |
| ����1 mol��ѧ����������/(kJ��mol��1) | 393 | 460 | 941 | 499 |
(II)��һ�Թ��м���0.01mol/L��KMnO4������Һ��0.1mol/LH2C2O4��Һ���ں����·������·�Ӧ��
2KMnO4+5 H2C2O4+3H2SO4��K2SO4+2MnSO4+10CO2+8H2O��5���Ӻ���Mn2+��Ũ��Ϊ0.004mol/L��
��4���Լ���0��5�����ڣ���(H2C2O4)��____________��
(5)�����Ӧ�ӿ�ʼ����һ��ʱ������ʡ�ʱ��ͼ����ͼ��
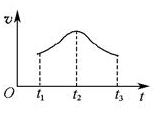 ���Խ���t1��t2��t2��t3���ʱ仯��ԭ��______________________________________________________��
���Խ���t1��t2��t2��t3���ʱ仯��ԭ��______________________________________________________�� ��֪��Ӧ��Fe(s)+CO2(g) FeO(s)+CO(g)����H=akJ��mol-1,ƽ�ⳣ��ΪK;
FeO(s)+CO(g)����H=akJ��mol-1,ƽ�ⳣ��ΪK;
��Ӧ��CO(g)+1/2O2(g) CO2(g)����H=bkJ��mol-1;
CO2(g)����H=bkJ��mol-1;
��Ӧ��Fe2O3(s)+3CO(g) 2Fe(s)+3CO2(g)����H=ckJ��mol-1������ڲ�ͬ�¶���,Kֵ����:
2Fe(s)+3CO2(g)����H=ckJ��mol-1������ڲ�ͬ�¶���,Kֵ����:
| �¶�/�� | 500 | 700 | 900 |
| K | 1.00 | 1.47 | 2.40 |
(1)��500 ��ʱ���з�Ӧ��,CO2����ʼŨ��Ϊ2 mol��L-1,CO��ƽ��Ũ��Ϊ����������
(2)��Ӧ��Ϊ��������(ѡ����ȡ����ȡ�)��Ӧ��
(3)700 ��ʱ��Ӧ�ٴﵽƽ��״̬,Ҫʹ��ƽ�������ƶ�,������������ʱ,���Բ�ȡ�Ĵ�ʩ����������(�����)��
A.��С��Ӧ����� B.ͨ��CO2
C.�¶����ߵ�900 �� D.ʹ�ú��ʵĴ���
E.����Fe����
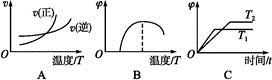
(4)����ͼ����Ϸ�Ӧ�ٵ�����������(�����)(ͼ��vΪ����,��Ϊ�������CO����,TΪ�¶���T1>T2)��
(5)�ɷ�Ӧ�ٺ͢ڿ����,��Ӧ2Fe(s)+O2(g)
 2FeO(s)�Ħ�H=����������
2FeO(s)�Ħ�H=���������� (6)�����ø�˹����д��Fe(����)��O2(����)�����õ�Fe2O3(����)���Ȼ�ѧ����ʽ:�� ��
 H2(g)+CO2(g) ��H����41kJ��mol��1
H2(g)+CO2(g) ��H����41kJ��mol��1 
 CH3OH(g) ��H1����90.7 kJ��mol��1
CH3OH(g) ��H1����90.7 kJ��mol��1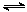 CH3OCH3(g)��CO2(g)�ġ�H�� kJ��mol-1��
CH3OCH3(g)��CO2(g)�ġ�H�� kJ��mol-1��
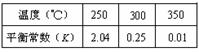
 ��Ӧ���̵������仯��ͼ��ʾ����֪l mol SO2(g)����Ϊ1 molSO3 (g)��
��Ӧ���̵������仯��ͼ��ʾ����֪l mol SO2(g)����Ϊ1 molSO3 (g)�� ����ش������⣻
����ش������⣻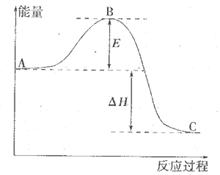
 ��������S(s)����2mol SO3(g)�ġ�H ��________________��
��������S(s)����2mol SO3(g)�ġ�H ��________________��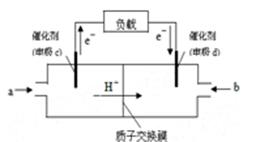
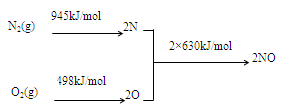
 2NO(g) ��H=���� ��
2NO(g) ��H=���� ��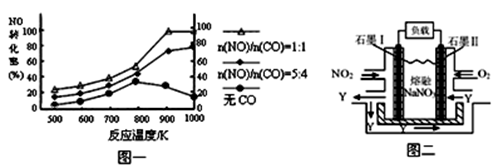
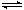 CH3OH(g)+H2O(g) ��H<0�ϳɼ״���
CH3OH(g)+H2O(g) ��H<0�ϳɼ״���