��Ŀ����
��I�����������ѳ�Ϊ��ǰ��δ����һ��ȫ���Կ��⡣Ϊ���Ŀǰȼ��ʹ�ù����еĻ�����Ⱦ���⣬��������ԴΣ�����е�ר���������̫���ܴ�ʹȼ��ѭ��ʹ�õĹ��룬��ͼ��ʾ��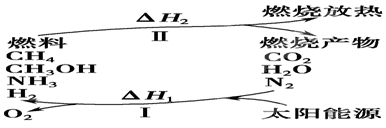
��ش��������⣺
(1)���̢������ת����ʽΪ________��ת��Ϊ________�ܡ�
(2)����ת�������У���H1�ͦ�H2�Ĺ�ϵ��________��
(3)����1 mol��ѧ��������������±���
| ���ۼ� | H��N | H��O | N��N | O��O |
| ����1 mol��ѧ����������/(kJ��mol��1) | 393 | 460 | 941 | 499 |
(II)��һ�Թ��м���0.01mol/L��KMnO4������Һ��0.1mol/LH2C2O4��Һ���ں����·������·�Ӧ��
2KMnO4+5 H2C2O4+3H2SO4��K2SO4+2MnSO4+10CO2+8H2O��5���Ӻ���Mn2+��Ũ��Ϊ0.004mol/L��
��4���Լ���0��5�����ڣ���(H2C2O4)��____________��
(5)�����Ӧ�ӿ�ʼ����һ��ʱ������ʡ�ʱ��ͼ����ͼ��
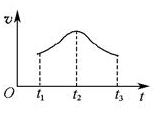 ���Խ���t1��t2��t2��t3���ʱ仯��ԭ��______________________________________________________��
���Խ���t1��t2��t2��t3���ʱ仯��ԭ��______________________________________________________��
��I����1��̫�� 1�֣���ѧ (1��)�� ��2����H1������H2 (1��)
��3��2N2(g)+6H2O(g)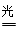 4NH3(g)+3O2(g) ��H��+1189kJ/mol
4NH3(g)+3O2(g) ��H��+1189kJ/mol (4��)
(4��)
����4��0.002md/(L��min) (2��)
��5��t1��t2˵��Mn2+��������t2��t3����Ӧ��Ũ�Ƚ��� (4��)
���������������I����1������ת��ʾ��ͼ��֪������I������̫���ܽ�������ˮ��CO2��ת��Ϊ�������������������״��ͼ���ȣ��������ת����̫����ת��Ϊ��ѧ�ܡ�
��2�����̢�����һ���������������������������״��ͼ����������ת��ΪCO2��ˮ�͵����ȣ����Ը��������غ��֪��H1������H2��
��3������ԭ���غ��֪��������ˮ��Ӧ���ɰ�����ͬʱ�����������ɣ���Ӧ�Ļ�ѧ����ʽΪ2N2+6H2O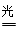 4NH3(g)+3O2�����ڷ�Ӧ�ȵ��ڶϼ����յ��������γɻ�ѧ�����ų��������IJ�ֵ����÷�Ӧ�ķ�Ӧ�ȡ�H��2��942kJ/mol��6��2��460kJ/mol��4��3��393kJ/mol��3��499kJ/mol����1189kJ/mol�����Ȼ�ѧ����ʽΪ2N2(g)+6H2O(g)
4NH3(g)+3O2�����ڷ�Ӧ�ȵ��ڶϼ����յ��������γɻ�ѧ�����ų��������IJ�ֵ����÷�Ӧ�ķ�Ӧ�ȡ�H��2��942kJ/mol��6��2��460kJ/mol��4��3��393kJ/mol��3��499kJ/mol����1189kJ/mol�����Ȼ�ѧ����ʽΪ2N2(g)+6H2O(g) 4NH3(g)+3O2(g) ��H��+1189kJ/mol��
4NH3(g)+3O2(g) ��H��+1189kJ/mol��
����4��5���Ӻ���Mn2+��Ũ��Ϊ0.004mol/L ������ݷ���ʽ��֪���IJ�������ʵ���Ũ����0.004mol/L�� ��0.01mol/L�����Է�Ӧ���ʦ�(H2C2O4)��0.01mol/L ��5min��0.002md/(L��min)��
��5������Ӱ�췴Ӧ���ʵ�����һ�����¶ȡ�Ũ�Ⱥʹ����ȣ���ϵ�¶Ȳ��䣬����Ӱ�췴Ӧ���ʵ����ؿ��Դ�Ũ�Ⱥʹ����ĽǶȷ�����t1��t2��Ӧ�������ߣ�˵��Mn2+���������ӿ��˷�Ӧ���ʣ���t2��t3��Ӧ�����ֽ��ͣ���˵����Ӧ��Ũ�Ƚ��͵��·�Ӧ���ʽ��͡�
���㣺���鷴Ӧ���������仯���Ȼ�ѧ����ʽ��д�Լ���Ӧ���ʼ������������Է�Ӧ���ʵ�Ӱ��

��1���ϳɰ���ҵ������������Ȼ����ˮ��Ӧ�Ʊ�������Ҫ��ӦΪ��
CH4(g)+ 2H2O(g)  CO2(g)+4H2(g)
CO2(g)+4H2(g)
��Ӧ�����������仯��ͼ��ʾ��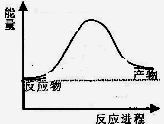
��÷�ӦΪ ��Ӧ������ȡ����ȡ���
����֪���ƻ�1mol��ѧ����Ҫ���յ��������±���ʾ��
| ��ѧ�� | C��H | O��H | C=O | H��H |
| ����������kJ/mol�� | a | b | c | d |
��2��ij�¶��£�10L�ܱ������г���2mol CH4��3mol H2O(g)������
CH4(g)+ 2H2O(g)
 CO2(g)+4H2 (g)��Ӧ�� ��Ӧ���е�4sʱCO2��Ũ��Ϊ0.08mol/L���ٹ�һ��ʱ�䷴Ӧ��ƽ�⣬ƽ��ʱ������ѹǿ����ʼʱ��1.4����
CO2(g)+4H2 (g)��Ӧ�� ��Ӧ���е�4sʱCO2��Ũ��Ϊ0.08mol/L���ٹ�һ��ʱ�䷴Ӧ��ƽ�⣬ƽ��ʱ������ѹǿ����ʼʱ��1.4������ �� ǰ4s��H2O(g)Ũ�ȱ仯��ʾ��ƽ����Ӧ����Ϊ���٣���4sʱ�����������H2���������Ϊ���٣��� ƽ��ʱ��CH4��Ũ���Ƕ��٣�
��Ҫ��д��������̣�
��14�֣�2013��10��������̨��������ܵ��ش���ʧ���м������Ľ����ɹ�����ҩƷ���������ֺ���Ҫ�����������������������и�Ч���������̼�����������Ҫ�ɷ�H2O2��һ����ɫճ��Һ�壬��ش��������⣺
�Ż�����䳣��Һ̬�£�N2H4��Ϊȼ�ϣ�Һ̬H2O2Ϊ��ȼ������֪��
N2H4��1��+O2��g��=N2��g��+2H2O��g�� ��H="-" 534 kJ��mol��1 ��
H2O2��1��=H2O��1��+1/2O2��g�� ��H="-" 98��64 kJ��mol��1 ��
H2O��1��=H2O��g�� ��H=+44kJ��mol��l ��
��ӦN2H4��1��+2H2O2��1��=N2��g��+4H2O��g���ġ�H= ��
�ƾݱ����������⻯����NaBH4(BԪ�صĻ��ϼ�Ϊ��3��)��H2O2�� ԭ�ϵ�ȼ �ϵ�أ��������ϲ���Pt/C���������ϲ���MnO2���������վ�ͨ�����ǵ磬�乤��ԭ����ͼ��ʾ��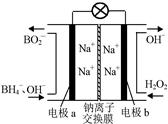
��õ�ص�������Ӧ____ ___
��H2O2��һ�ֲ��ȶ��ֽ�����ʡ���ͼ��H2O2��û�д���ʱ��Ӧ�����������仯ͼ������ͼ�ϻ���ʹ�ô����ӿ�ֽ�����ʱ���������ͼ ��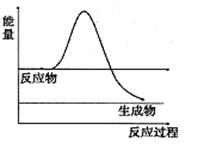
��ij��ѧ��ȤС�����ⶨH2O2�ķֽ����ʣ�ȡ��Һ0.5L���з����������������ʾ��
| t��S�� | 0 | 2 | 4 | 6 | 8 | 10 |
| n(H2O2) (moL) | 0.8 | 0.7 | 0.62 | 0.55 | 0.27 | 0.03 |
��H2O2����һ��ҩ�ﻯѧ��������������������ҩ��ķ�����

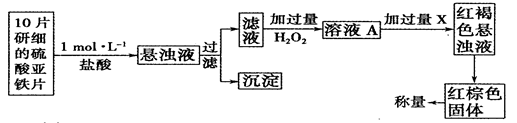
�ٴ˼�������мӹ���H2O2��Ӧ�����ӷ���ʽΪ______________��
�ڴӺ��ɫ������Һ�����ij����������������Ļ���������___________����������˳����д����
A������ B��ϴ�� C����ȡ D����Һ E����ȴ F������
������������ɫ���������Ϊ0��8960g����ô��ҩƬ��������������������Ϊ
_________��С������汣��һλ��Ч���֣���
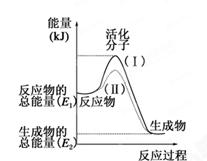
��ο�����ͼ������֪E1��134 kJ��mol��1��E2��368 kJ��mol��1������Ҫ��ش����⣺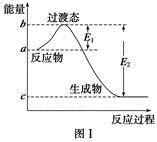
(1)ͼ����1 mol NO2(g)��1 mol CO(g)��Ӧ����CO2��NO�����е������仯ʾ��ͼ�����ڷ�Ӧ��ϵ�м����������Ӧ��������E1�ı仯�� (���������С�����䡱����ͬ)����H�ı仯�� ����д��NO2��CO��Ӧ���Ȼ�ѧ����ʽ�� ��
(2)�״����ӽ���Ĥȼ�ϵ���н��״�����ת��Ϊ���������ַ�Ӧ���Ȼ�ѧ����ʽ���£�
��CH3OH(g)��H2O(g)===CO2(g)��3H2(g) ��H����49.0 kJ��mol��1
��CH3OH(g)��O2(g)===CO2(g)��2H2(g) ��H����192.9 kJ��mol��1
��֪��H2O(g)===H2O(l) ��H����44 kJ��mol��1����״�����ȼ��ΪҺ̬ˮ���Ȼ�ѧ����ʽΪ ��
(3)�����ʾ�Dz��ֻ�ѧ���ļ��ܲ�����
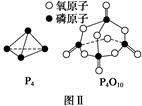
| ��ѧ�� | P��P | P��O | O===O | P===O |
| ����/kJ��mol��1 | a | b | c | x |
��֪����ȼ����Ϊd kJ��mol��1����������ȫȼ�յIJ���Ľṹ��ͼ����ʾ�������x�� kJ��mol��1(�ú�a��b��c��d�Ĵ�����ʽ��ʾ)��
 CH3OH(g) ��Hl= ��91kJ��mol��l
CH3OH(g) ��Hl= ��91kJ��mol��l ��Ӧ 3CO(g) +3H2(g) CH3OCH3(g) +CO2(g) ��H= .
��Ӧ 3CO(g) +3H2(g) CH3OCH3(g) +CO2(g) ��H= .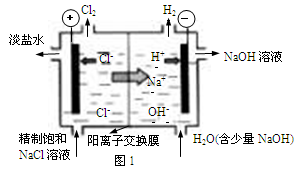

 Fe3����3H2O��ƽ�ⳣ��K�� ��
Fe3����3H2O��ƽ�ⳣ��K�� ��