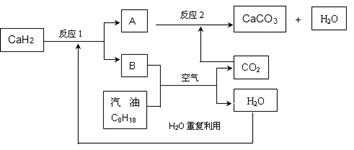
��Ŀ����
��16�֣�����β���dz��п�������Ҫ��Ⱦ��֮һ������Ҫ�к��ɷ���CO���������NOx���ȡ�
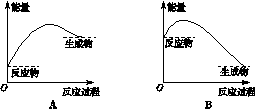
��1��NOx������ԭ��֮һ����������������ʱ����N2��O2��Ӧ���������仯ֵ����ͼ��ʾ��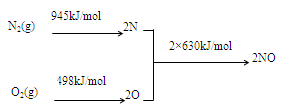
��N2(g)��O2(g) 2NO(g) ��H=���� ��
2NO(g) ��H=���� ��
��2������β����CO��NOx����Ч������Ϊ�����������Ҫ���⡣ij�о�С����ʵ������ij���ʹ�����CO��NO��ת�������о������NOת��ΪN2��ת�������¶ȡ�CO������ı仯�������ͼһ��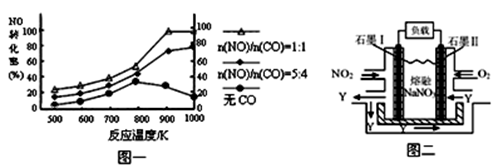
�� NO��CO���ʱ�����Ӧ�Ļ�ѧ����ʽΪ ��
�� 1000K��n(NO)/n(CO)=5:4ʱ��NO��ת����Ϊ75%����CO��ת����ԼΪ ��
�� ����n(NO)/n(CO)��ʵ�ʹ������Dz��ϱ仯�ģ���֤NOת���ʽϸߵĴ�ʩ�ǽ��¶ȴ�Լ������ K֮�䡣
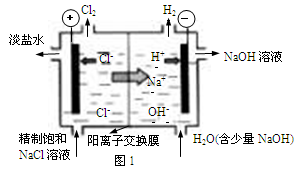
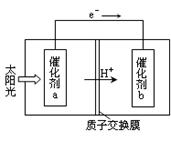
��3������β����NOx����ͨ��ȼ�ϵ��ʵ��ת�����Ѿ������� NO2��O2������NaNO3�Ƴ���ȼ�ϵ�أ���ԭ����ͼ����
�� ͼ��ʯī��Ϊ��ص� ����
�� �ڸõ��ʹ�ù����У�ʯīI�缫�ϵIJ�����������Y����缫��ӦʽΪ ��
��4���״�Ҳ������ȼ�ϵ�ء���ҵ�ϲ��÷�ӦCO2(g)+3H2(g)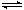 CH3OH(g)+H2O(g) ��H<0�ϳɼ״���
CH3OH(g)+H2O(g) ��H<0�ϳɼ״���
�� �ں����ܱշ�Ӧ���У�H2��ƽ��ת�������¶ȡ�ѹǿ�Ĺ�
ϵ��ͼ����ʾ����A��B��C���㴦��Ӧƽ�ⳣ��(KA��KB��KC)��
��С��ϵΪ ��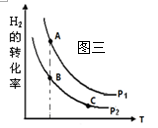
�� ij�����£���6molCO2��8molH2����2L�ܱ������з���
��Ӧ���ﵽƽ�����c(CO2)=2.0mol��L-1������¶��·�Ӧ��ƽ
�ⳣ��ֵΪ ��
(16�֣�ÿ��2��)
��1�� +183 kJ��mol��1��2�֣�δд��λ��δд��+������1�֣�
��2����2CO+2NO��N2 +2CO2��2�֣�δ��ƽ��1�֣��� ��93%����NOת������ý��Ϊ93.75%��������NO�����ֽ��Ӱ�죬ʵ��ֵ�������ֵ�в�࣬���Իش�93.75%�������ֵ�����֣� ��800��900
��3���� NO2��NO3�� ��e�� ��N2O5
��4����KA��KB>KC ��1/2
���������������1����ͼ��֪���������ӵĹ��ۼ�������Ҫ����945kJ���������������ӵĹ��ۼ�������Ҫ����498kJ����������2N��2O��ϳ�2��NO���ӷų�1260kJ�����������ԡ�H=945+498-1260=+183kJ/mol��
��2����NO��CO���ʱ�����ɵ����Ͷ�����̼����ѧ����ʽΪ2CO+2NO��N2 +2CO2��
��1000K��n(NO)/n(CO)=5:4ʱ��NO��ת����Ϊ75%����NO�����ʵ�����5mol����CO�����ʵ�����4mol,����NO�����ʵ�����5mol��75%=3.75mol��������CO �����ʵ�����3.75mol������CO��ת����Ϊ3.75mol/4mol��100%=93.75%��
����ͼһ��֪����n(NO)/n(CO)Ϊ��ֵ��800-900KʱNO��ת���ʽϸߣ�����900K��������CO����ʱ��NO��ת�����������ߣ�����COʱNO��ת����ȴ���½������������˵��¶���800-900K֮�䣻
��3������������ԭ��Ӧ������ͨ�������ļ��ǵ�ص�������ʯīIͨ����Ƕ����������壬����������Ӧ������������NԪ�صĻ��ϼ���+4�ۣ�NԪ�ػ��ϼ�����ֻ�����ߵ�+5�ۣ�����������Y��N2O5���缫��ӦʽΪNO2��NO3�� ��e�� ��N2O5
��4����A��B����¶���ͬ������ƽ�ⳣ����ͬ��C���¶ȸ���B���¶����ߣ�������Ӧ������ƽ�������С���������ߵĴ�С��ϵ��KA��KB>KC��
��6molCO2��8molH2����2L�ܱ������з���CO2(g)+3H2(g) CH3OH(g)+H2O(g) ƽ�����c(CO2)=2.0mol��L-1�������Ķ�����̼��Ũ����1mol/L,����������Ũ����3mol/L�����ɵļ״���ˮ������Ũ�ȶ���1mol/L,������ƽ��Ũ����mol/L,����K=c(CH3OH)c(H2O)/c(CO2)c(H2)3=1/2
CH3OH(g)+H2O(g) ƽ�����c(CO2)=2.0mol��L-1�������Ķ�����̼��Ũ����1mol/L,����������Ũ����3mol/L�����ɵļ״���ˮ������Ũ�ȶ���1mol/L,������ƽ��Ũ����mol/L,����K=c(CH3OH)c(H2O)/c(CO2)c(H2)3=1/2
���㣺���鷴Ӧ�ȵļ��㣬��ѧƽ�ⳣ�����жϼ����㣬�绯ѧ���۵�Ӧ��

��1���ϳɰ���ҵ������������Ȼ����ˮ��Ӧ�Ʊ�������Ҫ��ӦΪ��
CH4(g)+ 2H2O(g) 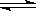 CO2(g)+4H2(g)
CO2(g)+4H2(g)
��Ӧ�����������仯��ͼ��ʾ��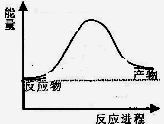
��÷�ӦΪ ��Ӧ������ȡ����ȡ���
����֪���ƻ�1mol��ѧ����Ҫ���յ��������±���ʾ��
| ��ѧ�� | C��H | O��H | C=O | H��H |
| ����������kJ/mol�� | a | b | c | d |
��2��ij�¶��£�10L�ܱ������г���2mol CH4��3mol H2O(g)������
CH4(g)+ 2H2O(g)
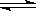 CO2(g)+4H2 (g)��Ӧ�� ��Ӧ���е�4sʱCO2��Ũ��Ϊ0.08mol/L���ٹ�һ��ʱ�䷴Ӧ��ƽ�⣬ƽ��ʱ������ѹǿ����ʼʱ��1.4����
CO2(g)+4H2 (g)��Ӧ�� ��Ӧ���е�4sʱCO2��Ũ��Ϊ0.08mol/L���ٹ�һ��ʱ�䷴Ӧ��ƽ�⣬ƽ��ʱ������ѹǿ����ʼʱ��1.4������ �� ǰ4s��H2O(g)Ũ�ȱ仯��ʾ��ƽ����Ӧ����Ϊ���٣���4sʱ�����������H2���������Ϊ���٣��� ƽ��ʱ��CH4��Ũ���Ƕ��٣�
��Ҫ��д��������̣�
CO�dz����Ļ�ѧ���ʣ��ڹ�ҵ��������;�ܹ㷺��
��1�� ��֪��ijЩ��Ӧ���Ȼ�ѧ����ʽ���£�
2H2��g��+SO2��g��=S��g��+2H2O��g�� ��H��+90.4kJ��mol��1
2CO��g��+O2��g��=2CO2��g�� ��H��-556.0kJ��mol��1
2H2��g��+O2��g��=2H2O��g�� ��H��-483.6kJ��mol��1
��д����CO��ȥ������SO2������S��g����CO2�Ȼ�ѧ����ʽ
��2�� ijȼ�ϵ����COΪȼ�ϣ��Կ���Ϊ��������������̬��K2CO3Ϊ����ʣ���д����ȼ�ϵ�������ĵ缫��Ӧʽ ��
��3����ij�¶��¡��ݻ���Ϊ2L�������ܱ������У�����ͬ��ʽͶ�뷴Ӧ����ֺ��º��ݣ�ʹ֮������Ӧ��2H2��g����CO��g�� CH3OH��g������H����dJ��mol��1��d��0������ʼͶ����������ﵽƽ��ʱ���й��������£�
CH3OH��g������H����dJ��mol��1��d��0������ʼͶ����������ﵽƽ��ʱ���й��������£�
| ʵ�� | �� | �� | �� |
| ��ʼͶ�� | 2 molH2��1 molCO | 1 mol CH3OH | 4 molH2��2 molCO |
| ƽ��ʱn��CH3OH �� | 0.5mol | n2 | n3 |
| ��Ӧ�������仯 | �ų�Q1kJ | ����Q2kJ | �ų�Q3kJ |
| ��ϵ��ѹǿ | P1 | P2 | P3 |
| ��Ӧ���ת���� | ��1 | ��2 | ��3 |
�����������еķ�Ӧ�ֱ��ƽ��ʱ�������ݹ�ϵ��ȷ���� ������ţ���
A����1����2��1 B��Q1��Q2��d
C����3����1 D��P3>2P1��2P2
E��n2��n3��1.0mol F��Q3��2Q1
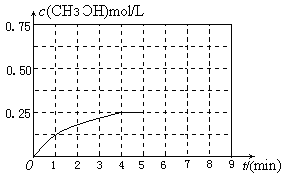
���������������������£�������������ϵ���ѹ����1L�����ڵ�8min�ﵽ�µ�ƽ�⣬�ӿ�ʼ����ƽ��ʱH2��ת����Ϊ65.5%��������ͼ�л�����5min ����ƽ��ʱCH3OH�����ʵ���Ũ�ȵı仯���ߡ�
��4��ʵ���ҳ��ü��ᣨһԪ�ᣩ���Ʊ�CO����֪25��ʱ��0.l mol/L����( HCOOH)��Һ��0.l mo1/L������Һ��pH�ֱ�Ϊ2.3��2.9��������ͬ���ʵ���Ũ�ȵ�����������Һ����HCOONa��Һ ��CH3COONa��Һ��Na2CO3��NaHCO3��Һ����pH�ɴ�С��˳���� ____����д��Һ��ţ�������0.l mo1/L HCOOH��Һ��0.l mo1/LHCOONa�������Ϻ����Һ������ȷ���� ____��
a��c(HCOO��)��c(HCOOH)��c��Na+����c��H+��
b��c(HCOO��)+c(HCOOH)=" 0.2" mo1/L
c��c(HCOO��)+2c(OH��)=c��HCOOH��+2c��H+��
d��c(HCOO��) ��c��Na+����c��H+����c(OH��)
 2PbSO4+2H2O�����ʱ��������Ӧʽ�� �ô�װ�õ��ˮ����ˮ��D2O����ɵĻ��Һ�����缫����Pt����ͨ��һ��ʱ������������ռ���33��6 L����״�������壬������Ϊ18.5 g������������Hԭ�Ӻ�Dԭ�Ӹ���֮�ȣ�
2PbSO4+2H2O�����ʱ��������Ӧʽ�� �ô�װ�õ��ˮ����ˮ��D2O����ɵĻ��Һ�����缫����Pt����ͨ��һ��ʱ������������ռ���33��6 L����״�������壬������Ϊ18.5 g������������Hԭ�Ӻ�Dԭ�Ӹ���֮�ȣ�  CH3OH(g) ��Hl= ��91kJ��mol��l
CH3OH(g) ��Hl= ��91kJ��mol��l ��Ӧ 3CO(g) +3H2(g) CH3OCH3(g) +CO2(g) ��H= .
��Ӧ 3CO(g) +3H2(g) CH3OCH3(g) +CO2(g) ��H= .