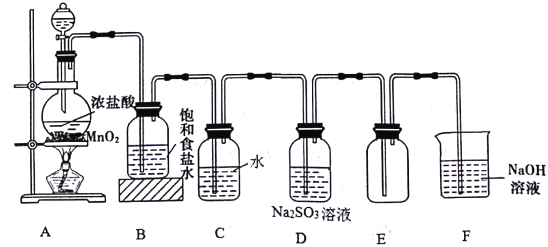
��Ŀ����
����Ŀ��ij��ɫ��Һ�п��ܺ���Mg2+��Ba2+��Cl-��HCO3-�е�һ�ֻ������ӡ�Ϊȷ����ɷ֣���������ʵ�飺
ʵ��1��ȡl0mL��ɫ��Һ���μ�����ϡ��������������
ʵ��2����ȡl0mL��ɫ��Һ������������Na2SO4��Һ���а�ɫ�������ɡ�
ʵ��3����ʵ��l�����Һ����ƿ�У�����ƿ����μ���NaOH��Һ���μӹ����в������������������NaOH��Һ������Ĺ�ϵ��ͼ��ʾ��

�ش��������⣺
��1��ԭ��Һ�в����ڵ�������________�����ڵ�������___________________��
��2��ʵ��3�У�ͼ����OA�η�Ӧ�����ӷ���ʽΪ___________________��
��3������ͼ�����ԭ��Һ��Mg2+�����ʵ���Ũ��______________����д��������̣�
���𰸡� HCO3- Mg2+��Ba2+��Cl- H++OH-= H2O 1 mol/L
��������ʵ��1��ȡl0mL��ɫ��Һ���μ�����ϡ��������������˵��ԭ��Һ�в�����HCO3-��ʵ��2����ȡl0mL��ɫ��Һ������������Na2SO4��Һ���а�ɫ�������ɡ�˵����Һ����Ba2+��ʵ��3����ʵ��l�����Һ����ƿ�У�����ƿ����μ���NaOH��Һ���μӹ����в������������������NaOH��Һ������Ĺ�ϵͼ��������ʼ����˵����Һ����H+���ٲ����˳�����˵����Һ���� Mg2+������Һ�ʵ����ԣ���Һ��һ����������Cl-��
��1��ԭ��Һ�в����ڵ�������HCO3-�����ڵ�������Mg2+��Ba2+��Cl- ����2��ʵ��3�У�ͼ����OA���к���Һ��H+����Ӧ�����ӷ���ʽΪH++OH-= H2O����3������ͼ�����ԭ��Һ��Mg2+�����ʵ���Ũ��Ϊ��n(Mg(OH)2)=0.58g/58g��mol��1=0.01mol,c(Mg2��)=0.01mol/0.01L=1mol��L��1��

����Ŀ������ǿ������ʼ��ǵ���ʵ������ȫ��ȷ���ǣ� ��
A | B | C | D | |
ǿ����� | NaCl | H2SO4 | CaCO3 | HNO3 |
������� | HF | BaSO4 | HClO | CH3COOH |
�ǵ���� | Cl2 | CO2 | H2S | SO2 |
A.AB.BC.CD.D