��Ŀ����
19��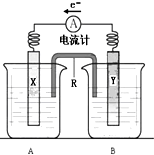 ��ͼ��ͭпԭ���װ�ã����ص��ܷ�Ӧ�ǣ�
��ͼ��ͭпԭ���װ�ã����ص��ܷ�Ӧ�ǣ�Zn��s��+CuSO4��aq���TZnSO4��aq��+Cu��s��
��ش��������⣺
��1��R�����������ţ�����������ͨ�ڵ�·��R�е�����������A����A��B���е���Һ��
��2���缫X�IJ�����Cu��B�еĵ������Һ��ZnSO4��Һ��
��3��YΪԭ��صĸ�������缫��Ӧʽ��Zn-2e-�TZn2+��
��4�����ӣ�Ni-Cd���ɳ�������ִ��������й㷺Ӧ�ã���֪ij���ӵ�صĵ������ҺΪKOH��Һ����䡢�ŵ簴��ʽ���У�Cd+2NiOOH+2H2O$?_{���}^{�ŵ�}$Cd��OH��2+2Ni��OH��2
�ŵ�ʱ��������������ҺpH����С������������С�������ʱ�������ĵ缫��ӦʽΪNi��OH��2-e-+OH-�TNiOOH+H2O��
���� ͭпԭ����У�п�ϻ��ã�Ϊԭ��صĸ���������Zn-2e-=Zn2+��ͭΪ����������Cu2++2e-=Cu��ԭ��ع���ʱ�����Ӵӵ�ظ��������·�����������Դ˽��1����2����3���⣻
��4���������ǵ��أ�����ʧ���ӷ���������Ӧ��Ԫ�ػ��ϼ����ߣ��ŵ�ʱԭ��أ�������ʧ���ӷ���������Ӧ���缫���������������ӳ������ݴ˽�ɣ�
��� �⣺��1���ɵ��������֪XΪ������YΪ��������װ��ͼ��֪RΪ���ţ�����ʹ�����ձ��е���Һ����ͨ·���������������ƶ������������ƶ����ʴ�Ϊ�����ţ���ͨ�ڵ�·�� A��
��2��XΪ��������Cu��⣬��Һ�е�ͭ���ӱ���ԭ���������ҺӦΪZnSO4 ��Һ���ʴ�Ϊ��Cu��ZnSO4��Һ��
��3��YΪ������������������һ��������������Ӧ���缫����ʽΪZn-2e-�TZn2+���ʴ�Ϊ������Zn-2e-�TZn2+��
��4���ŵ�ʱ�����Ϸ���������Ӧ��Cd-2e-+2OH-=Cd��OH��2�缫������Һ�ļ��Լ�����pH��С�����ʱ��������������Ӧ���缫��Ӧ��Ni��OH��2+OH--e-��NiOOH+H2O���ʴ�Ϊ����С��Ni��OH��2-e-+OH-�TNiOOH+H2O��
���� ���⿼���˵��غ�ԭ��ص�ԭ��Ӧ�ã�����ת������Ҫ�ǵ缫���ơ��缫��Ӧ���缫�жϣ�����ԭ��غ͵��صĹ���ԭ���ǹؼ���

| A�� | 0.1Lmol��L-1���������Һ�У�c��NH4+��+c��H+����c��SO42-��+c��OH-�� | |
| B�� | ����������Ƶ�pH=7�Ļ����Һ�У�c��CH3COO-��+c��CH3COOH����c��Na+�� | |
| C�� | ��pH=5��H2SO4��Һϡ��1��103����c��H+����c��SO42-��=2��1 | |
| D�� | �����£�pH=4��NaHC2O4��Һ�У�c��C2O42-����c��H2C2O4�� |
| A�� | ��λʱ��������n molO2��ͬʱ����2n mol NO2 | |
| B�� | ��λʱ��������n molO2��ͬʱ����2n mol NO2 | |
| C�� | NO2�ķֽ����ʵ���O2���γ����� | |
| D�� | ��Ӧ����������Ũ����� |
| A�� | ���ֿڷ�Һ���ḻ�ĵ����ס�п����Ԫ�� | |
| B�� | ���������в����κλ�ѧ���� | |
| C�� | ��������ˮ���Դ��������в����κ����� | |
| D�� | û��ˮ��û������ |
| A�� | Na2CO3 KCl | B�� | NaCl KCl | C�� | CaCl2 NaCl | D�� | K2CO3 CaCl2 |
| NO��NO2�� | NH4Cl��AlCl3�� | FeCl2��FeCl3�� | NaNO3��NH4NO3�� | |
| ��ѡ�Լ���ѧʽ | ||||
| ����������� |
 B��
B�� C��
C�� D��
D��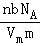
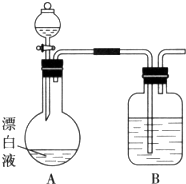 ʵ��С��ͬѧ����һ����ij��ֽ���۳���Ư��Һ��NaCl��NaClO�Ļ��Һ������ʢ�ű���KAl��SO4��2��Һ�ij��У�����ж��¼�����С��ͬѧΪ̽���ж�ԭ�����������ʵ�飮
ʵ��С��ͬѧ����һ����ij��ֽ���۳���Ư��Һ��NaCl��NaClO�Ļ��Һ������ʢ�ű���KAl��SO4��2��Һ�ij��У�����ж��¼�����С��ͬѧΪ̽���ж�ԭ�����������ʵ�飮