��Ŀ����
����Ŀ��A��G�������ʵ�ת����ϵ��ͼ��ʾ�����ַ�Ӧ�����ͷ�Ӧ����δ����������У�A��BΪ��ѧ��ѧ�����Ľ������ʣ�C�ǵ���ɫ���壬D������ǿ����Һ��Ӧ��������ǿ����Һ��Ӧ��F��Һ�м���AgNO3��Һ����������ϡ����İ�ɫ������E��G��ɫ��Ӧ���ʻ�ɫ���١��ܾ�Ϊȼ�շ�Ӧ��
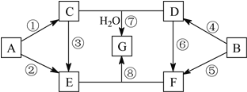
��ش��������⣺
(1)D�Ļ�ѧʽ____________��G�Ļ�ѧʽ____________��
(2)��Ҫ��д�����з�Ӧ�ķ���ʽ��
�۵Ļ�ѧ����ʽ��___________________��
B��E����Һ��Ӧ�����ӷ���ʽ��________________��
(3)��E����Һ��μ��뵽F����Һ������������������_____���ܷ�Ӧ�����ӷ���ʽΪ_______��
���𰸡�Al2O3 NaAlO2 2Na2O2��2H2O=4NaOH��O2�� 2Al��2OH-��2H2O=2AlO2-��3H2�� �Ȳ�����ɫ��״���������������ܽ� Al3+��4OH��=AlO2-��2H2O
��������
A����ѧ��ѧ�����Ľ������ʣ�A����ȼ�շ�Ӧ����C��C�ǵ���ɫ���壬CΪNa2O2��AΪNa��BΪ��ѧ��ѧ�����Ľ������ʣ�B����ȼ�շ�Ӧ����D��D������ǿ����Һ��Ӧ��������ǿ����Һ��Ӧ��DΪAl2O3��BΪAl��C+D+H2O��G��G����ɫ��Ӧ�ʻ�ɫ��GΪNaAlO2��A��E��C��E�Լ�E����ɫ��Ӧ�ʻ�ɫ��E�к�NaԪ�أ�F��Һ�м���AgNO3��Һ����������ϡ����İ�ɫ������F��Һ�к�Cl-�����D��F��E+F��G����EΪNaOH��FΪAlCl3��
A����ѧ��ѧ�����Ľ������ʣ�A����ȼ�շ�Ӧ����C��C�ǵ���ɫ���壬CΪNa2O2��AΪNa��BΪ��ѧ��ѧ�����Ľ������ʣ�B����ȼ�շ�Ӧ����D��D������ǿ����Һ��Ӧ��������ǿ����Һ��Ӧ��DΪAl2O3��BΪAl��C+D+H2O��G��G����ɫ��Ӧ�ʻ�ɫ��GΪNaAlO2��A��E��C��E�Լ�E����ɫ��Ӧ�ʻ�ɫ��E�к�NaԪ�أ�F��Һ�м���AgNO3��Һ����������ϡ����İ�ɫ������F��Һ�к�Cl-�����D��F��E+F��G����EΪNaOH��FΪAlCl3��
��1�������Ϸ�����֪D�Ļ�ѧʽΪAl2O3��G�Ļ�ѧʽΪNaAlO2��
�ʴ�Ϊ��Al2O3��NaAlO2��
��2���۵Ļ�ѧ����ʽ��2Na2O2��2H2O=4NaOH��O2��
B��E����Һ��Ӧ�����ӷ���ʽ��2Al��2OH-��2H2O=2AlO2-��3H2��
�ʴ�Ϊ��2Na2O2��2H2O=4NaOH��O2����2Al��2OH-��2H2O=2AlO2-��3H2����
��3��EΪNaOH��FΪAlCl3����E����Һ��μ��뵽F����Һ���������������ķ�Ӧ����ΪAl3++3OH-=Al(OH)3��Al(OH)3+OH-=AlO2-+2H2O���������Ȳ�����ɫ��״���������������ܽ⣬�ܷ�Ӧ���ӷ���ʽΪAl3+��4OH��=AlO2-��2H2O��
�ʴ�Ϊ���Ȳ�����ɫ��״���������������ܽ⣻Al3+��4OH��=AlO2-��2H2O��

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�