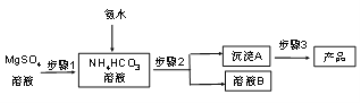
��Ŀ����
����Ŀ�������仯�����ڹ�ũҵ������������������ҪӦ�á�
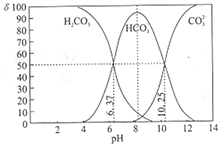
(1)�Ȱ�[NH2Cl(g)]��һ�ֳ�Ч���ͺ���������,�л������־õ�ɱ������,��д����ɱ����������ˮ��Ӧ�Ļ�ѧ����ʽ________________��
(2)�ǰ�(NH2OH)���л��ϳɵij�����ԭ������ҵ�Ͽ������ᣮ����ˮ��Һ������ʽ��е��,�ڹ��缫��NO3-��ת��ΪNH2OH,�Բ�Ϊ��һ��,��õ�ⷴӦ���ܻ�ѧ����ʽΪ________________��
(3)������Һ�Ǽ���ȩ������Ҫ�Լ�,����Һ�д���Ag(NH3)2+(aq)![]() Ag+(aq)+2NH3(aq)��������,��ӦAgCl(s)+2NH3(aq)
Ag+(aq)+2NH3(aq)��������,��ӦAgCl(s)+2NH3(aq)![]() Ag(NH3)2+(aq)+Cl-(aq)�Ļ�ѧƽ�ⳣ��K=1.94��10-3,��Ksp(AgCl)=1.76��10-10,��K[Ag(NH3)2+]=________________��
Ag(NH3)2+(aq)+Cl-(aq)�Ļ�ѧƽ�ⳣ��K=1.94��10-3,��Ksp(AgCl)=1.76��10-10,��K[Ag(NH3)2+]=________________��
(4)ˮ����(N2H4��H2O)��������¯ˮ�ij�������N2H4��H2OΪ��Ԫ�����ˮ��Һ�е�һ�����뷽��ʽ��ʾΪN2H4��H2O![]() N2H5++OH-��������(N2H6Cl2)��һ�ֿ������Σ����һ��ˮ������ӷ���ʽΪ________________,��Һ������Ũ���ɴ�С������˳��Ϊ________________��
N2H5++OH-��������(N2H6Cl2)��һ�ֿ������Σ����һ��ˮ������ӷ���ʽΪ________________,��Һ������Ũ���ɴ�С������˳��Ϊ________________��
(5)����һ����Ҫ�ĵ��ʡ���ҵ�ϳɰ���ӦΪ��![]() N2(g)+
N2(g)+ ![]() H2(g)
H2(g)![]() NH3(g),�÷�Ӧ�Ļ��Ea1=254kJ/mol��
NH3(g),�÷�Ӧ�Ļ��Ea1=254kJ/mol��
�ٲ�������,��ػ�ѧ�������������£�
��ѧ�� | H-H | N | N-H |
E/kJ��mol-1 | 436 | 946 | 391 |
��ӦNH3(g)![]()
![]() N2(g)+
N2(g)+ ![]() H2(g)�Ļ��Ea2=________________��
H2(g)�Ļ��Ea2=________________��
��һ��������,1molN2��3.6molH2���ܱ������г�ַ�Ӧ,��÷ų�������Ϊ13.8kJ,��H2��ת����Ϊ________________,Ϊ���ԭ�ϵ�������,��ҵ�ϳɰ�Ӧ�ò�ȡ�Ĵ�ʩ��=________________��
���𰸡� NH2Cl+H2O![]() HClO+NH3(��NH2Cl+2H2O
HClO+NH3(��NH2Cl+2H2O![]() HClO+NH3��H2O) 2HNO3+2H2O
HClO+NH3��H2O) 2HNO3+2H2O![]() 2NH2OH+3O2�� 9.07��10-8 N2H62+
2NH2OH+3O2�� 9.07��10-8 N2H62+![]() N2H5++H+ c(Cl-)>c(N2H62+)>c(H+)>c(N2H5+)>c(OH-) 300kJ/mol 12.5% ѭ������(���������𰸾��÷�)
N2H5++H+ c(Cl-)>c(N2H62+)>c(H+)>c(N2H5+)>c(OH-) 300kJ/mol 12.5% ѭ������(���������𰸾��÷�)
����������1���Ȱ���ˮ������Ӧ��NH2-��ˮ���������H+��ϳɰ�����Cl+��OH-�������ǿ�����Ե�����HClO���÷�Ӧ�Ļ�ѧ����ʽΪNH2Cl+H2O![]() HClO+NH3(��NH2Cl+2H2O
HClO+NH3(��NH2Cl+2H2O![]() HClO+NH3��H2O)����ȷ�𰸣�NH2Cl+H2O
HClO+NH3��H2O)����ȷ�𰸣�NH2Cl+H2O![]() HClO+NH3(��NH2Cl+2H2O
HClO+NH3(��NH2Cl+2H2O![]() HClO+NH3��H2O)��
HClO+NH3��H2O)��
(2)NO3-��ת��ΪNH2OH,������ԭ��Ӧ��������ӦΪ2NO3-+12e-+14H+ =2NH2OH+4H2O����������Ӧ��12OH--12e-=3O2��+6H2O����������Ӧ��ӣ��õ��õ�ⷴӦ���ܻ�ѧ����ʽΪ2HNO3+2H2O![]() 2NH2OH+3O2������ȷ�𰸣�2HNO3+2H2O
2NH2OH+3O2������ȷ�𰸣�2HNO3+2H2O![]() 2NH2OH+3O2����
2NH2OH+3O2����
(3)��ѧƽ�ⳣ��K=1.94��10-3=c(Ag(NH3)2+)��c(Cl-)/c2(NH3)= c(Ag(NH3)2+)��c(Cl-)��c(Ag+)/c2(NH3) ��c(Ag+)=1.76��10-10��c(Ag(NH3)2+)/c2(NH3)��������c(Ag(NH3)2+)/c2(NH3)=1.94��10-3/1.76��10-10��K[Ag(NH3)2+]= c2(NH3)/ c(Ag(NH3)2+)=1.76��10-10/1.94��10-3=9.07��10-8����ȷ����9.07��10-8��
(4)N2H4��H2OΪ��Ԫ�������������(N2H6Cl2)Ϊǿ�������Σ�ˮ���Լ��ԣ����һ��ˮ������ӷ���ʽΪN2H62+![]() N2H5++H+ ����Һ������Ũ���ɴ�С������˳��Ϊc(Cl-)>c(N2H62+)>c(H+)>c(N2H5+)>c(OH-)����ȷ�𰸣�N2H62+
N2H5++H+ ����Һ������Ũ���ɴ�С������˳��Ϊc(Cl-)>c(N2H62+)>c(H+)>c(N2H5+)>c(OH-)����ȷ�𰸣�N2H62+![]() N2H5++H
N2H5++H
(5) �ٰ��ֽⷴӦ����H=3��391-1/2��436-3/2��946=+46 kJ��mol-1����Ӧ��(��H)��������Ӧ�Ļ�����淴Ӧ�Ļ��֮���H=Ea1-Ea2=254-Ea2=+46��Ea2=300kJ��mol-1����ȷ�𰸣�300 kJ��mol-1��
�ڸ�������������֪��N2(g)+ 3H2(g)![]() 2NH3(g)����H=-92 kJ��mol-1����Ӧ�ų�������Ϊ13.8kJʱ��������������Ϊ3��13.8/92=0.45 mol, H2��ת����Ϊ0.45/3.6��100%=12.5%��Ϊ���ԭ�ϵ�������,��ҵ�ϳɰ�Ӧ�ò�ȡ�Ĵ�ʩ�ǹ�ҵ�ϳɰ�Ϊ�����ԭ�ϵ������ʣ�Ҫ����ѭ��������Ҳ���ǰ�δ��Ӧ�ĵ����������ӷ�Ӧ������з�������������ͻط�Ӧ���У����ںϳɰ������������С�ķ�Ӧ��Ϊ���ԭ�������ʣ�Ҳ���ʵ�����Ӧ��ϵѹǿ����ȷ����12.5% �� ѭ������(���������𰸾��÷�)��
2NH3(g)����H=-92 kJ��mol-1����Ӧ�ų�������Ϊ13.8kJʱ��������������Ϊ3��13.8/92=0.45 mol, H2��ת����Ϊ0.45/3.6��100%=12.5%��Ϊ���ԭ�ϵ�������,��ҵ�ϳɰ�Ӧ�ò�ȡ�Ĵ�ʩ�ǹ�ҵ�ϳɰ�Ϊ�����ԭ�ϵ������ʣ�Ҫ����ѭ��������Ҳ���ǰ�δ��Ӧ�ĵ����������ӷ�Ӧ������з�������������ͻط�Ӧ���У����ںϳɰ������������С�ķ�Ӧ��Ϊ���ԭ�������ʣ�Ҳ���ʵ�����Ӧ��ϵѹǿ����ȷ����12.5% �� ѭ������(���������𰸾��÷�)��

����Ŀ����������������Ӧ����㷺�����������ͺ����²��ϵ�Ӧ�ñ��ܹ�ע��
(1)��¯������ұ��������Ҫ��������������Ҫ��Ӧ��:
��Ӧ | ��H(kJ/mol) | K |
i. Fe2O3(s)+3C(s) | +489 | K1 |
ii. Fe2O3(s)+3CO(g) | X | k2 |
iii. C(s)+CO2(g) | +172 | k3 |
�Լ��㣬X=____��K1��K2��K3֮��Ĺ�ϵΪ__________________��
(2)T1��ʱ����ij�����ܱ������м���һ������Fe2O3��C��������Ӧi����Ӧ�ﵽƽ�����t1ʱ�̣��ı�ij������V(��)��ʱ��(t)�ı仯��ϵ��ͼ1��ʾ����t1ʱ�̸ı������������_______(��д��ĸ)��
a.�����¶Ȳ��䣬ѹ������ b.����������䣬�����¶�
c.����������䣬������̼�� d.�����¶�������䣬����COŨ��
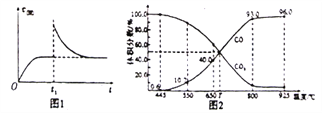
(3)��һ���¶��£���ij����ɱ�ĺ�ѹ�ܱ�����(P��)����1molCO2�������̼��������Ӧiii��ƽ��ʱ��ϵ����������������¶ȵĹ�ϵ��ͼ2��ʾ��
��650��ʱ���÷�Ӧ��ƽ������յ�������__________��(����ʱ�������¶ȶԡ�H��Ӱ��)
��T��ʱ������ƽ����ϵ���ٳ���һ������v(CO2):V(CO)=5:4�Ļ�����壬ƽ��______(�������������)�ƶ���
��925��ʱ����ƽ���ѹ����ƽ��Ũ�ȱ�ʾ�Ļ�ѧƽ�ⳣ��KpΪ_____��[�����ѹ(p��)=������ѹ(p��)�������������ij���ʵ�ƽ���ѹ�������ʵ���Ũ��Ҳ���Ա�ʾ��ѧƽ�ⳣ��������Kp]
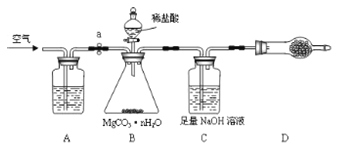
(4)��ԭ���ԭ�����Գ�ȥ���Է�ˮ�е�������ϩ��AsO3-����ԭ����ͼ3��ʾ(������ڲ�Ϊ���������)��
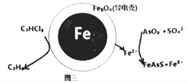
�ڳ��۹����У�����������е�FeΪԭ��ص�____��(���������)��д��C2HCl3������汻��ԭΪ����ĵ缫��ӦʽΪ____________________��
(5)NaHS��������ˮ�����ij���������֪:25��ʱ����ӦHg2+(aq)+HS-(aq) ![]() HgS(s)+H+(aq)��ƽ�ⳣ��K=1.75��1038��H2S�ĵ���ƽ�ⳣ��Ka1=1.0��10-7��Ka2=7.0��10-15��
HgS(s)+H+(aq)��ƽ�ⳣ��K=1.75��1038��H2S�ĵ���ƽ�ⳣ��Ka1=1.0��10-7��Ka2=7.0��10-15��
��NaHS�ĵ���ʽΪ____________________��
��Ksp(HgS)=_______________��