��Ŀ����
����Ŀ��I.��ͼ����ѧ��ѧ���õ�ʵ��װ�ã�ڽ�ش���������
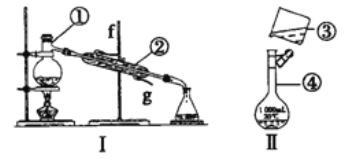
��1����ͼI�������ʵķ����ᴿ���÷��뷽��Ϊ________��������װ��I����ƾ���ˮ�Ļ�����ȱ�ٵ�����__________������������__________�����Ľ�ˮ����________(����f" ����g��)��
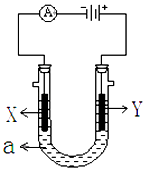
��2����������500mL��0.1mol/LKCl ��Һ��װ������ijͬѧת����Һ��ʾ��ͼ��ָ��ͼ�еĴ���֮��: ��_________����____________��
��3��������~������ʹ��ʱ�������Ƿ�©ˮ����_________(�����)��
��.������ᴿ���ʵķ�����������ʵ���о����ҹ㷺Ӧ���ڹ�ҵ����������ʵ�ʡ�
��4����ˮɹ�ι����У�ͨ�������ķ����ɵõ����ι������ٽ����ι����ܽ�����ù��˵ķ�����ȥ���е���ɳ�������г���ɳ����п���������(���±���ʾ)���ɰ��±���ʾ�������µ�˳�����γ�ȥ(��������д����)��
���� | �����Լ��Ļ�ѧʽ | ������Ӧ�����ӷ���ʽ |
������ | ___________ | ___________ |
MgCl2 | NaOH | ___________ |
CaCl2 | Na2CO3 | ___________ |
���μ��������Լ�����ȫ��Ӧ���ٽ��й��ˣ��ټ������������� �ɳ�ȥ������������� �������ӡ�
��5�� �Ӻ����п���ȡ�ⵥ�ʡ�����ȡ�ķ����ɽ��ⵥ�ʴ�ˮ��Һ����ȡ�������÷���������I2�ڲ�ͬ�ܼ��е�_______�Բ�ͬ��
���𰸡� ���� �¶ȼ� ������ƿ g δ�ò��������� δ����500ml������ƿ �� BaCl2 Ba2����SO42��=BaSO4�� Mg2++2OH-=Mg(OH)2�� CO32- + Ca2+ = CaCO3�� ��Ba2+ + CO32- = BaCO3�� �ܽ�
��������I.��1����װ��I�������Ȼ�̼�;ƾ��Ļ�����ȱ�ٵ��������¶ȼƣ�������������������е�ʵ�����������Ϊ��������������������ƿ�����Ľ�ˮ����g����2����������500mL��0.1mol/LKCl��Һ��Ӧѡ��500mL����ƿ����ת��Һ��ʱû���ò�����������װ�����еĴ���֮��:��δ�ò�������������δ����500ml������ƿ����3���������ӡ�����������ʹ��ʱ��Ҫ����Ƿ�©ˮ��������~������ʹ��ʱ�������Ƿ�©ˮ���� �ܣ���.��4������������ñ����ӳ���������������Ȼ�����Һ���Խ���������ӳ�����Ba2����SO42��=BaSO4�������������Ƴ�ȥþ���ӣ�Mg2++2OH-=Mg��OH)2�� ����������̼������ӳ������������Ӽ��������̼������Һת��Ϊ���������Ǽ���̼������ҺҪ���ڼ�����Ȼ�����Һ֮������̼���ƻ��ȥ��Ӧʣ����Ȼ�������ȫ��Ӧ���ٽ��й��ˣ�����ټ��������ȥ��Ӧʣ���̼������ӡ������������ӡ���5���Ӻ����п���ȡ�ⵥ�ʡ�����ȡ�ķ����ɽ��ⵥ�ʴ�ˮ��Һ����ȡ�������÷���������I2�ڲ�ͬ�ܼ��е��ܽ��Բ�ͬ��

 ��ǿ��У��ĩ���100��ϵ�д�
��ǿ��У��ĩ���100��ϵ�д� �óɼ�1��1��ĩ���100��ϵ�д�
�óɼ�1��1��ĩ���100��ϵ�д� ��״Ԫ���źþ�ϵ�д�
��״Ԫ���źþ�ϵ�д�