��Ŀ����
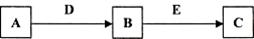
A��J����ѧ��ѧ�г��������ʣ�����֮���ת����ϵ���¿�ͼ��ʾ�����ֲ�������ȥ������֪A��һ�ָ��۵����ʣ�J��һ�ֺ��ɫ������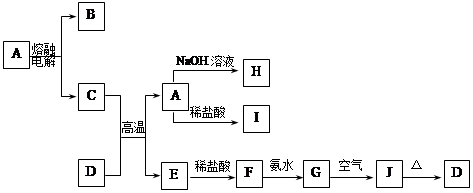
��ش��������⣺
��1��A�Ļ�ѧʽΪ ����ˮ�е��ܽ��� ��ѡ����ܡ��������ܡ��������ܡ�����
��2��H��Һ��ͨ�������CO2���䷴Ӧ�����ӷ���ʽ�� ��
G��J�Ļ�ѧ����ʽΪ ��
��Ӧ�������� ��
��3��D����ǡ������һ������ϡ������ú��ʵĻ�ѧ�����ʾ������Һ�����Ե�ԭ�� ��
��1��Al2O3 ������
��2��AlO2-+CO2+H2O�TAl(OH)3��+HCO3-,4Fe(OH)2+2H2O+O2�T4Fe(OH)3��
��3��Fe3++3H2O?Fe(OH)3+3H+
���������������1��A��һ�ָ��۵����ʣ����ڸ����µ�⣬ӦΪ����������BΪO2��D��һ�ֺ���ɫ���壬ӦΪFe2O3����Al�������ȷ�Ӧ����EΪFe�����ݷ�Ӧ��ϵ��֪HΪNaAlO2��IΪAlCl3��FΪFeCl2��GΪFe(OH)2��JΪFe(OH)3����A�Ļ�ѧʽΪAl2O3 ��ˮ�е��ܽ���Ϊ���ܡ�
��2������������֪HΪNaAlO2��GFe(OH)2��Ϊ��H��Һ��ͨ�������CO2�����ӷ���ʽ��AlO2-+CO2+H2O�TAl(OH)3��+HCO3-��G��J�Ļ�ѧ����ʽΪΪ4Fe(OH)2+2H2O+O2�T4Fe(OH)3����Ӧ����Ϊ�ɰ�ɫ��Ϊ����ɫ�����ձ�Ϊ���ɫ������
��3��D����ǡ������һ������ϡ����������Ȼ�����Һ����ˮ����Һ�����ԣ���Fe3++3H2O?Fe(OH)3+3H+���ʴ�Ϊ��Fe3++3H2O?Fe(OH)3+3H+��
���㣺Ԫ�ؼ�����������ʼ��ƶϡ�����ˮ��

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д���ʵ�����ijͬѧȡһС�����������ˮ��Ӧ��ʵ�顣������������⣺
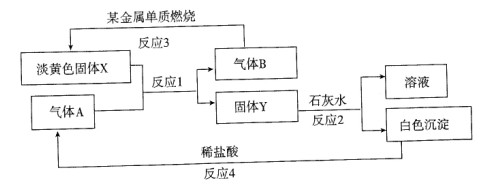
�п��Ľ����Ʊ�¶�ڿ����У����ȹ۲쵽�������� ����������Ӧ�Ļ�ѧ����ʽ�� ��
(2)����Ͷ��ˮ�к����ڻ���һ��С������һ�������ܵó��Ľ����ǣ�
�� ���� ��
��һС����Ͷ��ʢ�б���ʯ��ˮ���ձ��У������ܹ۲쵽�������� �����ţ���
| A������������ | B�����ڻ���С����Һ�����ζ� |
| C����Һ�ײ�������ɫ�Ľ��������� | D����Һ����� |
��4����������ʵ������������������йر仯����˵���������Ʊ�����ú���е�Ŀ���� ��
��1���֣�
һλͬѧ�ڸ�ϰʱ��������һ��ϰ�⣺ij��ɫ��Һ�п��ܺ��С�H+��OH-��Na+��NO3-�����������ۺ�ֻ����H2���ʸ���ɫ��Һ���ܴ��������ļ������ӡ�
��1���������۲���H2��˵��������______��������ԡ���ԭ�ԡ�����
��2����ͬѧ��������H+�������ڣ���NO3-�Ͳ��ܴ������ڡ�
���ʵ��֤ʵ���£�
| װ �� | �� �� |
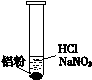 | ��. ʵ���ʼ��δ���������� ��. ��һ������������ݣ�Һ���Ϸ���dz��ɫ ��. �Թܱ��ȣ���Һ���� |
�� �����ܽ�Al2O3��Ĥ�����ӷ���ʽ��______��
�� ���������Ʋ���Һ�в�����NO��Ϊ��һ��ȷ�ϣ���������ʵ�飺
| ʵ �� | �� �� | �� �� |
| ʵ��1 | ��ʪ��KI��������ֽ���ڿ����� | δ���� |
| ʵ��2 | ��ʪ��KI��������ֽ����dz��ɫ���� | ��ֽ���� |
b. ʵ��1��Ŀ����_______��
c. ʵ��1��2˵����Ӧ������NO��������NO�����ӷ���ʽ����������
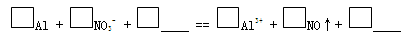
��3���ټ��裺��OH-�������ڣ�NO3-Ҳ���ܲ��ܴ������ڡ�
�������ʵ��֤ʵ���£�
| װ �� | �� �� |
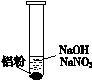 | ��. ʵ���ʼ��δ���������� ��. ��һ������������ݣ��д̼�����ζ |
Ϊȷ�ϡ��̼�����ζ�����壬��������ʵ�飺��ʪ��KI��������ֽ���飬δ��������ʪ���ɫʯ����ֽ���飬��ֽ������
�� �̼�����ζ��������______��
�� ��������������ӷ���ʽ��______��
��4����NaOH��Һ�м������ۣ����ֻ�������H2���ɣ��仯ѧ����ʽ��______��
��5��ʵ����֤ʵ��NO3?���ᡢ���Ի����ж���һ���������ԣ������������ʣ��������������ϰ���е���ɫ��Һһ���ܴ������ڵ���Na+��OH-��