��Ŀ����
19�������ơ���һ�ֳ��õ�ѧϰ����������ʱ���������Ľ��ۣ��������ƽ�������ȷ���ǣ�������| A�� | IVA��Ԫ���⻯���۵�˳���ǣ�GeH4��SiH4��CH4����VA��Ԫ���⻯���۵�˳��Ҳ�ǣ�AsH3��PH3��NH3 | |
| B�� | �ڶ�����Ԫ���⻯����ȶ���˳���ǣ�HF��H2O��NH3�����������Ԫ���⻯����ȶ���˳��Ҳ�ǣ�HCl��H2S��PH3 | |
| C�� | ��ҵ���õ���Ȼ�þ�Ʊ�þ���ʣ���ҵ��Ҳ���õ���Ȼ����Ʊ������� | |
| D�� | Fe3O4�ɸ�д�����������ʽΪ��FeO•Fe2O3�����Pb3O4Ҳ�ɸ�дΪ��PbO•Pb2O3 |
���� A��IVA��Ԫ���⻯�����Է�����Խ���۵�Խ�ߣ�����VA��Ԫ���⻯���а������백����֮���������������۵��쳣�ߣ�
B���ǽ�����Խǿ�⻯��Խ�ȶ���
C���Ȼ����ǹ��ۻ��������ʱ�����磻
D��Pb�ڻ������еĻ��ϼ���+2�ۡ�+4�ۣ���д������ʱҪ��ѭ���ϼ۲��䡢ԭ���غ㣮
��� �⣺A��IVA��Ԫ���⻯�����Է�����Խ���۵�Խ�ߣ������۵�˳���ǣ�GeH4��SiH4��CH4������VA��Ԫ���⻯���а������백����֮���������������۵��쳣�ߣ�����NH3��AsH3��PH3����A����
B���ǽ�����Խǿ�⻯��Խ�ȶ���ͬ�����зǽ����ԣ�F��O��N�������⻯����ȶ���˳���ǣ�HF��H2O��NH3������������Ԫ�أ�Cl��S��P�������⻯����ȶ���˳��Ҳ�ǣ�HCl��H2S��PH3����B��ȷ��
C���Ȼ����ǹ��ۻ��������ʱ�����磬��ҵ�Ͽ��õ���������Ʊ�������C����
D����ΪPb�ڻ������еĻ��ϼ���+2�ۡ�+4�ۣ�����Pb3O4д���������������ʽΪ2PbO��PbO2��������PbO•Pb2O3����D����
��ѡB��
���� ���⿼����֪ʶ��϶࣬�漰�⻯���۵�ߵ͵��жϡ��⻯���ȶ��Ե��жϡ�������ұ��������ȣ������ڻ���֪ʶ�Ŀ��飬��Ŀ�ѶȲ���

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�| A�� | ֻ����C��H����Ԫ�� | |
| B�� | ֻ����C��O����Ԫ�� | |
| C�� | ����C��H��O����Ԫ�� | |
| D�� | һ������C��H����Ԫ�أ�����ȷ���Ƿ���OԪ�� |
| A�� | ����Һ�����ԣ���c��K+��=c��CH3COO-�� | |
| B�� | ����Һ�ʼ��ԣ���c��K+����c��CH3COO-�� | |
| C�� | ����Һ�����ԣ���c��H+����c��CH3COO-����c��K+�� | |
| D�� | ��Һ�ʵ����ԣ���c��H+��+c��K+��=c��CH3COO-��+c��OH-�� |
| A�� | pH=3�������pH=5������������ϣ�pH=4 | |
| B�� | �����£�pH=9�ļ�����Һ�У����ܴ���CH3COOH���� | |
| C�� | ������Һ�б�Ȼ��c��H+��=c��OH-��=1��10-7 mol/L | |
| D�� | ��0.1 mol/L HCl��Һ�м�������������ˮ����Һ�и������ӵ����ʵ���Ũ�Ⱦ���С |
| A�� | A��B��C�ķ�����֮��Ϊ1��3��2 | |
| B�� | A��B��C��Ũ����� | |
| C�� | ��λʱ������ nmol A��ͬʱ���� 3nmol B | |
| D�� | ����C��������C�ֽ��������� |
| A�� | CH4���ӵĽṹʽ��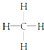 | B�� | ��ϩ�Ľṹ��ʽ��CH2CH2 | ||
| C�� | F-�ṹʾ��ͼ��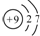 | D�� | �����ӵĵ���ʽ��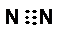 |
| A�� | ���ȩ������ԭ����Ư�ۡ���������������ͬ | |
| B�� | 1 mol ���ȩ���ӿɱ�1 mol Cu��OH��2��ȫ���� | |
| C�� | CH3CH�TCHCH2COOH�����ȩ��Ϊͬ���칹�� | |
| D�� | 10g���ȩ��ȫȼ��������0.6 mol O2 |
| A�� | 100mL 2mol/L NH4NO3��Һ | B�� | 40mL 0.5mol/L Ca��NO3��2��Һ | ||
| C�� | 50mL 1.5mol/L Al��NO3��3��Һ | D�� | 150mL 1mol/L Mg��NO3��2��Һ |
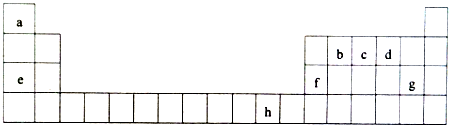
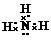 ��������cԭ�ӵ��ӻ����������sp3�ӻ���Ca3�ر�������a2d����ԭ����NH3��H2O֮�����γ������3��h2+������Ca3����λ���γ�[h��Ca3��4]2+���ӣ���ˮ��Һ�з��ܡ�����ȫ���룮
��������cԭ�ӵ��ӻ����������sp3�ӻ���Ca3�ر�������a2d����ԭ����NH3��H2O֮�����γ������3��h2+������Ca3����λ���γ�[h��Ca3��4]2+���ӣ���ˮ��Һ�з��ܡ�����ȫ���룮