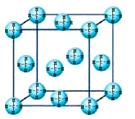
��Ŀ����
��12�֣�
����10��Ԫ�ص����ʡ��������±����У����Ǿ�Ϊ������Ԫ�ء�
�ش��������⣺
��1��D��Ԫ�������� ��
H��Ԫ�ط����� ��
B��Ԫ�����ڱ��е�λ���ǣ����ڡ��壩
��2��������Ԫ���γɵ�����������ˮ�����У�������ǿ�Ļ�����ķ���ʽ�� ��
������F2A2�ĵ���ʽ�ǣ� �����ɸ����ʵĻ�ѧ������Ϊ________________
��3���õ���ʽ��ʾA�ļ��⻯����γɹ������£� ��
G���⻯��ĽṹʽΪ ��
��4��һ�������£�IA2������������A���ʳ�ַ�Ӧ����20g��̬������ų�24.6 kJ������д�����Ȼ�ѧ����ʽ ��
��5����JԪ�صĵ�����AԪ�صĵ��ʿ����Ƴɵ�أ������װ��KOHŨ��Һ���ö�Ķ��Ե缫���ҽ���KOH��Һ���ڼ�ͨ��J�ĵ��ʣ��Ҽ�ͨ��A�ĵ��ʣ�����ĵ缫��ӦʽΪ��____________________��
����10��Ԫ�ص����ʡ��������±����У����Ǿ�Ϊ������Ԫ�ء�
| | A | B | C | D | E | F | G | H | I | J |
| ԭ�Ӱ뾶(10��10m) | 0.74 | 1.60 | 1.52 | 1.10 | 0.99 | 1.86 | 0.75 | 0.82 | 0.102 | 0.037 |
| ������ ���ϼ� | | ��2 | ��1 | ��5 | ��7 | ��1 | ��5 | ��3 | +6 | +1 |
| ��2 | | | ��3 | ��1 | | ��3 | | -2 | |
��1��D��Ԫ�������� ��
H��Ԫ�ط����� ��
B��Ԫ�����ڱ��е�λ���ǣ����ڡ��壩
��2��������Ԫ���γɵ�����������ˮ�����У�������ǿ�Ļ�����ķ���ʽ�� ��
������F2A2�ĵ���ʽ�ǣ� �����ɸ����ʵĻ�ѧ������Ϊ________________
��3���õ���ʽ��ʾA�ļ��⻯����γɹ������£� ��
G���⻯��ĽṹʽΪ ��
��4��һ�������£�IA2������������A���ʳ�ַ�Ӧ����20g��̬������ų�24.6 kJ������д�����Ȼ�ѧ����ʽ ��
��5����JԪ�صĵ�����AԪ�صĵ��ʿ����Ƴɵ�أ������װ��KOHŨ��Һ���ö�Ķ��Ե缫���ҽ���KOH��Һ���ڼ�ͨ��J�ĵ��ʣ��Ҽ�ͨ��A�ĵ��ʣ�����ĵ缫��ӦʽΪ��____________________��
��1���ף�1�֣���B��1�֣����������ڵڢ�A�� ��1�֣�
��2��HClO4��1�֣�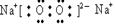 ��1�֣� ���Ӽ����Ǽ��Լ���2�֣�
��1�֣� ���Ӽ����Ǽ��Լ���2�֣�
(3)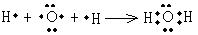 ��1�֣�
��1�֣� 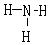 ��1�֣�
��1�֣�
(4)2 SO2 (g)+O2(g)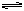 2SO3(g)�� ��H =��196.8 kJ/mol��2�֣�
2SO3(g)�� ��H =��196.8 kJ/mol��2�֣�
(5) H2 �C 2e- + 2OH- ="=" 2H2O��1�֣�
��2��HClO4��1�֣�
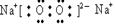 ��1�֣� ���Ӽ����Ǽ��Լ���2�֣�
��1�֣� ���Ӽ����Ǽ��Լ���2�֣�(3)
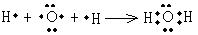 ��1�֣�
��1�֣� 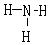 ��1�֣�
��1�֣�(4)2 SO2 (g)+O2(g)
 2SO3(g)�� ��H =��196.8 kJ/mol��2�֣�
2SO3(g)�� ��H =��196.8 kJ/mol��2�֣�(5) H2 �C 2e- + 2OH- ="=" 2H2O��1�֣�
��

��ϰ��ϵ�д�
 ��ʦ������Ԫ��ĩ���100��ϵ�д�
��ʦ������Ԫ��ĩ���100��ϵ�д� ��У������Ԫͬ��ѵ��������ϵ�д�
��У������Ԫͬ��ѵ��������ϵ�д�
�����Ŀ

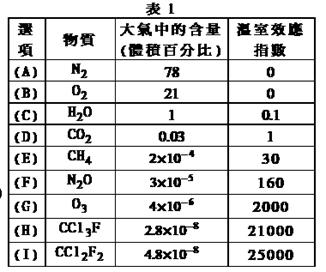
 ���칹
���칹