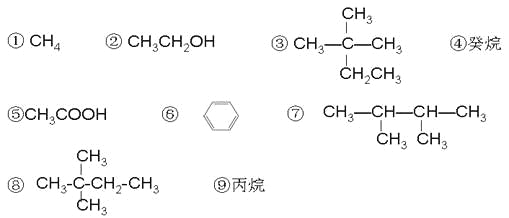
��Ŀ����
����Ŀ������п����п������Ҫ�Ļ�������ԭ�ϡ�
��1��ZnO �� Al2O3 �Ļ�ѧ�������ƣ�ZnO �� NaOH ��Һ��ת����[Zn(OH)4]2�����ӷ���ʽΪ_____________��
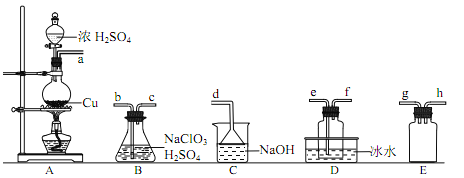
��2������п�õ�������п�к���Ǧ��ͭ�����ʣ��ᴿ�������£�
![]()
![]()
����ͼ�еġ������Ϊ________���ѧʽ����
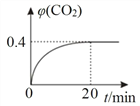
��ij�¶�ʱ���ڷ�Ӧ��ķ�Ӧ¯�У���ʼʱ c(CO)Ϊ 0.3 molL1����Ӧ������ CO2 ��������� ��(CO2)��ͼ��ʾ����Ӧ���ƽ�ⳣ�� K��_____��
�����д�ʩ��������߷�Ӧ���� ZnO ת���ʵ���________��
a������ ZnO ��Ͷ���� b���ʵ���ѹ c����п������ʱ����
�ܷ�Ӧ���У�ÿת�� 1mol ���ӣ���Ӧ���� 174 kJ���� H2��_____________��
��3���ⶨ����п��Ʒ���ȣ���ȡ 0.5000g ��Ʒ�����ܺ����� 250 mL ����ƿ�У�ҡ�ȡ���ȡ 25.00 mL ����Һ���� 0.04000 molL1 �� EDTA��Na2H2Y����Һ�ζ����е� Zn2+����Ӧ����ʽΪ Zn2+��H2Y2��ZnY2��2H+�����ʲ���Ӧ����ƽ�еζ����Σ�ƽ������ EDTA ��Һ 15.12mL��
�����ζ���δ�� EDTA ��Һ��ϴ���ⶨ�����___���ƫ�ߡ�����ƫ�͡����䡱����
����Ʒ����Ϊ��________________���г�����ʽ���ɣ���
��4���ʵ�ӫ�����е���ɫӫ��ۺ��� ZnS�������� 0.05mol ZnS ��ӫ������� 500mL�����У���ȫ�ܽ����Һ�� c(S2)��__________ molL1������֪��Ksp(ZnS)��2.5��1023��������Һ����ı仯��
���𰸡� ZnO+2OH-+H2O=[Zn(OH)4]2- Zn 0.4mol/L c -696kJ/mol ƫ�� ![]() 2.5��10-22
2.5��10-22
��������(1)ZnO��Al2O3�Ļ�ѧ�������ƣ����������������Ʒ�Ӧ����ƫ�����ƣ���������п���������Ƶķ�Ӧ�ķ���ʽΪ��ZnO+H2O+2OH-=[Zn(OH)4]2-���ʴ�Ϊ��ZnO+H2O+2OH-=[Zn(OH)4]2-��
(2)����Ӧ����ZnO(s)+CO(g)Zn(g)+CO2(g)��п��������Ϊ�����������Ϊ����п���ʴ�Ϊ��Zn��
��ij�¶�ʱ���ڷ�Ӧ���ķ�Ӧ¯�У���ʼʱc(CO)Ϊ0.3molL-1����Ӧ�����дﵽƽ��CO2�����������(CO2)��ͼ��ʾΪ0.4��
ZnO(s)+CO(g)Zn(g)+CO2(g)
��ʼ��(mol/L) 0.3 0
�仯��(mol/L) x x
ƽ����(mol/L) 0.3-x x
![]() =0.4��x=0.12��ƽ�ⳣ��K=
=0.4��x=0.12��ƽ�ⳣ��K=![]() =0.67���ʴ�Ϊ��0.67��
=0.67���ʴ�Ϊ��0.67��
��a������ZnO��Ͷ����������пΪ���岻Ӱ��ƽ�⣬����пת���ʲ��䣬��a����b����Ӧǰ������������䣬�ʵ���ѹ����Ӱ��ƽ���ƶ�����b����c����п������ʱ���룬ƽ�������������пת��������c��ȷ���ʴ�Ϊ��c��
����Ӧ����2Zn(g)+O2(g)�T2ZnO(s)����Ӧ��2molZn��ȫ��Ӧ����ת��4mol����Ӧÿת��1mol���ӣ���Ӧ����174kJ��ת��4mol���ӷ�Ӧ����696KJ����Ӧ�ʱ���H=-696KJ/mol���ʴ�Ϊ��-696KJ/mol��
(3)���ζ���δ��EDTA��Һ��ϴ���ڲ�ˮĤ��ϡ�ͱ���Һ�����ı���Һ��������ⶨ���ƫ�ߣ��ʴ�Ϊ��ƫ�ߣ�
����ȡ0.5000g��Ʒ�����ܺ�����250mL����ƿ�У�ҡ�ȣ���ȡ25.00mL����Һ����0.04000molL-1��EDTA(Na2H2Y)��Һ�ζ����е�Zn2+(��Ӧ����ʽΪZn2++H2Y2-�TZnY2-+2H+�����ʲ���Ӧ)��ƽ�еζ����Σ�ƽ������EDTA��Һ15.12mL��
Zn2++H2Y2-�TZnY2-+2H+��
1 1
n 15.12��10-3L��0.04000mol/L
n(ZnO)=n(Zn2+)=15.12��10-3L��0.04000mol/L��250mL��Һ��n(ZnO)=15.12��10-3L��0.04000mol/L��![]() ����Ʒ����=
����Ʒ����=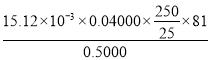 ��100%���ʴ�Ϊ��
��100%���ʴ�Ϊ��  ��100%��
��100%��
(4)������0.05molZnS��ӫ�������500mL�����У���ȫ�ܽ����Һ��п����Ũ��c(Zn2+)=![]() =0.1mol/L��Ksp(ZnS)=2.5��10-23=c(Zn2+)c(S2-)��
=0.1mol/L��Ksp(ZnS)=2.5��10-23=c(Zn2+)c(S2-)��
c(S2-)��2.5��10-22mol/L���ʴ�Ϊ��2.5��10-22��

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�