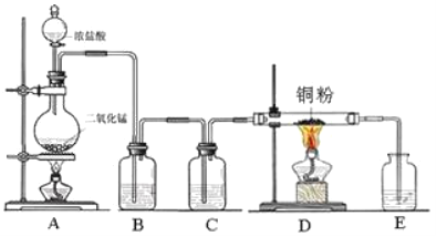
题目内容
【题目】铬钾矾[KCr(SO4)2˙12H2O]在鞣革、纺织等工业上有广泛的用途。某学习小组用还原K2Cr2O7,酸性溶液的方法制备铬钾矾,装置如图所示:
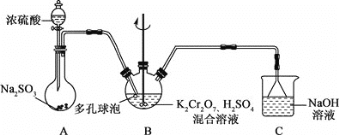
回答下列问题:
(1)A中盛装浓硫酸的仪器名称是________。
(2)装置B中反应的离子方程式为_________________________________。
(3)反应结束后,从B装置中获取铬钾矾晶体的方法是:向混合物中加入乙醇后析出晶体,过滤、洗涤、低温干燥,得到产品。
①加入乙醇后再进行过滤的目的是____________。
②低温干燥的原因是_________________________。
(4)铬钾矾样品中铬含量的测定:取mg样品置于锥形瓶中用适量水溶解,加入足量的Na2O2进行氧化,然后用硫酸酸化使铬元素全部变成Cr2O72-,用c mol/L的FeSO4溶液滴定至终点,消耗FeSO4溶液v mL,该样品中铬的质量分数为________。
【答案】分液漏斗 Cr2O72-+3SO2 +2H+=3SO42-+2Cr3++7H2O 降低铬钾矾在溶液中的溶解量,有利于晶体析出 防止铬钾矾失去结晶水 ![]() %
%
【解析】
由装置图可知,A为制备还原剂二氧化硫的装置,B为制备铬钾矾的装置,C为尾气处理装置。B中发生二氧化硫和Cr2O72-的反应,生成铬离子和硫酸根离子,根据氧化还原反应的规律,结合化学实验的基本操作分析解答。
(1)根据装置图,A中盛装浓硫酸的仪器为分液漏斗,故答案为:分液漏斗;
(2)装置B中二氧化硫和Cr2O72-的反应,生成铬离子和硫酸根离子,反应的离子方程式为Cr2O72-+3SO2 +2H+=3SO42-+2Cr3++7H2O,故答案为:Cr2O72-+3SO2 +2H+=3SO42-+2Cr3++7H2O;
(3)反应结束后,从B装置中获取铬钾矾晶体的方法是:向混合物中加入乙醇后析出晶体,过滤、洗涤、低温干燥,得到产品。
①加入乙醇降低铬钾矾在溶液中的溶解量,有利于晶体析出,然后过滤即可,故答案为:降低铬钾矾在溶液中的溶解量,有利于晶体析出;
②由于铬钾矾晶体[KCr(SO4)2˙12H2O]中含有结晶水,高温下会失去结晶水,为了防止铬钾矾失去结晶水,实验中需要低温干燥,故答案为:防止铬钾矾失去结晶水;
(4)将样品置于锥形瓶中用适量水溶解,加入足量的Na2O2进行氧化,然后用硫酸酸化使铬元素全部变成Cr2O72-,用c mol/L的FeSO4溶液滴定至终点,根据得失电子守恒,存在Cr2O72-~6 FeSO4,根据Cr2O72-+3SO2 +2H+=3SO42-+2Cr3++7H2O有2Cr3+~ Cr2O72-~6 FeSO4,消耗FeSO4溶液v mL,消耗的FeSO4的物质的量=c mol/L×v ×10-3L= c v×10-3 mol,因此样品中含有Cr3+ 的物质的量=![]() ×c v×10-3 mol,样品中铬的质量分数=
×c v×10-3 mol,样品中铬的质量分数=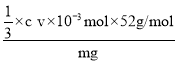 ×100%=
×100%=![]() %,故答案为:
%,故答案为:![]() %。
%。

【题目】下表是元素周期表的一部分,针对表中的①~⑩种元素,请用化学用语回答下列问题:
族 周期 | IA | IIA | IIIA | IVA | VA | VIA | VIIA | 0 |
2 | ⑥ | ⑦ | ⑧ | |||||
3 | ① | ③ | ⑤ | ⑨ | ⑩ | |||
4 | ② | ④ |
(1) 在③~⑦元素中,原子半径最大的是__________(填元素符号);
(2) ⑦元素的最高价氧化物对应的水化物与其氢化物能生成盐M,M中含有的化学键类型有________________________;
(3)写出元素①和⑧的单质在加热条件下反应生成的化合物的电子式:_____________。
(4) ③、⑤、⑦、⑧形成的离子,其半径由小到大的顺序是________(填离子符号)
(5) ①~⑨中元素最高价氧化物对应的水化物中酸性最强的是_____________(填物质化学式),呈两性的氢氧化物是_________(填物质化学式);该化合物与NaOH溶液反应的离子方程式为___________。
(6) 用电子式表示元素③与⑨形成化合物的过程_____________________________。
(7)写出工业冶炼⑤的化学方程式:_______________________________________。