��Ŀ����
����Ŀ�����Ŵ�����Ⱦ���������أ�����������ʮ�������ڼ䣬����������(SO2)�ŷ�������8%����������(NOx)�ŷ��Լ���10%��������̼(CO2)���ŷ���ҲҪ������١�
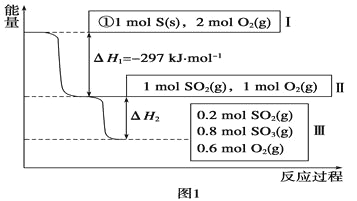
��1���ں��£��ݻ�Ϊ1L�����У�����Է������½ϻ����䷴Ӧ���̺�������ϵ��ͼ1��ʾ(��֪��2SO2(g)��O2(g) ![]() 2SO3(g) ��H����196.6 kJ��mol��1)����ش��������⣺
2SO3(g) ��H����196.6 kJ��mol��1)����ش��������⣺
��д���ܱ�ʾ���ȼ���ȵ��Ȼ�ѧ����ʽ��_________��,
����H2��_________kJ��mol��1��
������ͬ�����£�����1molSO3��0.5mol��O2����ﵽƽ��ʱSO3��ת����Ϊ_______����ʱ�÷�Ӧ______(�����ų�������������)_______kJ��������
��2���й�������ŵ����2020�꣬��λGDP������̼�ŷű�2005����40%~50%��
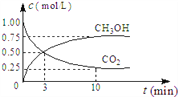
��CO2��ת�����л���ʵ��̼ѭ�������ݻ�Ϊ1L���ܱ������У�����lmolCO2��3molH2��һ�������·�Ӧ:CO2(g)+3H2(g) ![]() CH3OH(g)+H2O(g) ��H=-49.0kJ/mol,���CO2��CH3OH(g)Ũ����ʱ��仯��ͼ��ʾ����3min��9min��v(H2)=_______mol/(L��min)
CH3OH(g)+H2O(g) ��H=-49.0kJ/mol,���CO2��CH3OH(g)Ũ����ʱ��仯��ͼ��ʾ����3min��9min��v(H2)=_______mol/(L��min)
��Ϊ�˼ӿ컯ѧ��Ӧ������ʹ��ϵ����������ʵ������٣�������������ʱ���ɲ�ȡ�Ĵ�ʩ��_______ (����)��
A.�����¶� B.��С������� C.�ٳ���CO2���� D.ʹ�ú��ʵĴ���
��3����ҵ�ϣ�CH3OHҲ����CO2��H2�ϳɡ��ο��ϳɷ�ӦCO(g)+2H2(g) ![]() CH3OH(g)��ƽ�ⳣ��������˵����ȷ����______________��
CH3OH(g)��ƽ�ⳣ��������˵����ȷ����______________��
�¶�/�� | 0 | 100 | 200 | 300 | 400 |
ƽ�ⳣ�� | 667 | 13 | 1.9��10-2 | 2.4��10-4 | 1��10-5 |
A.�÷�Ӧ����Ӧ�Ƿ��ȷ�Ӧ
B.�÷�Ӧ�ڵ����²����Է����У������¿��Է����У�˵���÷�Ӧ��S<0
C.��T��ʱ��1L�ܱ������У�Ͷ��0.1molCO��0.2molH2,�ﵽƽ��ʱ��COת����Ϊ50%�����ʱ��ƽ�ⳣ��Ϊ100
D.��ҵ�ϲ����Ըߵ�ѹǿ(5MPa)��250��,����Ϊ�������£�ԭ����ת�������
���𰸡� S(s) +O2(g)=SO2(g) ��H=-297 kJ/mo1 78.64 20% ���� 19.66 0.125 B AC
����������1����ȼ������ָ1mol��ȼ����ȫȼ�������ȶ�������ų��������������ȼ���ȵ��Ȼ�ѧ����ʽΪS(s) + O2(g)��SO2(g)��H=-297kJ/mol��
������2SO2(g)��O2(g)![]() 2SO3(g) ��H����196.6 kJ��mol��1������0.8mol SO3�ų�������Ϊ78.64 kJ�������H2����78.64kJ��mol��1���ʴ���78.64��
2SO3(g) ��H����196.6 kJ��mol��1������0.8mol SO3�ų�������Ϊ78.64 kJ�������H2����78.64kJ��mol��1���ʴ���78.64��
������1molSO3��0.5molO2��ȫת��ΪSO2��O2������ԭ���ij�ʼ����ͬ����������ƽ���ǵ�Чƽ�⣬��ƽ��ʱSO2Ϊ0.2mol��SO3Ϊ0.8mol������SO3ת����0.2mol����ﵽƽ��ʱSO3��ת����Ϊ![]() ��100%=20%����֪2SO2��g��+O2��g��=2SO3��g����H=-196.6kJ��mol-1������Ӧ�������ʱ��Ӧ�ȴ�С���䣬�����෴������SO3ת��ΪSO2ʱ��Ӧ���ȣ�����Ϊ196.6��
��100%=20%����֪2SO2��g��+O2��g��=2SO3��g����H=-196.6kJ��mol-1������Ӧ�������ʱ��Ӧ�ȴ�С���䣬�����෴������SO3ת��ΪSO2ʱ��Ӧ���ȣ�����Ϊ196.6��![]() =19.66 kJ���ʴ��ǣ�20%�����գ�19.66��
=19.66 kJ���ʴ��ǣ�20%�����գ�19.66��
��2������3min��9min��v��CO2��=��c��CO2������t=��0.50mol/L0.25mol/L����6min����v��H2��=3v��CO2��=0.125mol/(L��min)���ʴ��ǣ�0.125��
��A������Ӧ���ȣ������¶ȷ�Ӧ���ʼӿ죬ƽ�����淴Ӧ������У���ϵ����������ʵ������ӣ�A����
B����С�������ѹǿ����Ӧ���ʼӿ죬ƽ��������Ӧ������У���ϵ����������ʵ������٣�B��ȷ��
C���ٳ���CO2���巴Ӧ���ʼӿ죬ƽ��������Ӧ������У���ϵ����������ʵ������ӣ�C����
D��ʹ�ú��ʵĴ������ܸı�ƽ��״̬��D������
�ʴ�ѡB��
��3��A.���ݱ������ݿ�֪�����¶�����ƽ�ⳣ����С��������ӦΪ���ȷ�Ӧ��A��ȷ��
B.��A��֪����ӦΪ���ȷ�Ӧ����H��0������Ӧ�������С�ģ�����S��0��������G=��H-T��S��0��֪�÷�Ӧ�ڵ��������Է����У�B����
C�����ƽ������ʽ����ʽ���㣬ƽ�ⳣ������������ƽ��Ũ����֮�����Է�Ӧ��ƽ��Ũ����֮����
CO��g��+2H2��g��![]() CH3OH��g��
CH3OH��g��
��ʼ����mol/L�� 0.1 0.2 0
�仯����mol/L�� 0.05 0.1 0.05
ƽ������mol/L�� 0.05 0.1 0.05
ƽ�ⳣ��K=![]() =100��C��ȷ��
=100��C��ȷ��
D������ƽ��������У��������Ǵ������������ԭ������ת���ʸߣ�D����
�ʴ�ѡAC��

����Ŀ����1����һ���Ϊ10 L���ܱ������У�ͨ��һ������CO��H2O����850 ��ʱ������Ӧ��CO(g)��H2O(g)![]() CO2(g)��H2(g)����H<0��CO��H2OŨ�ȱ仯��ͼ��ʾ����0��4 min��ƽ����Ӧ����v(CO)��_____________ mol/(L��min)��
CO2(g)��H2(g)����H<0��CO��H2OŨ�ȱ仯��ͼ��ʾ����0��4 min��ƽ����Ӧ����v(CO)��_____________ mol/(L��min)��

��2��t��(����850 ��)ʱ������ͬ�����з���������Ӧ�������ڸ����ʵ�Ũ�ȱ仯�����
ʱ��(min) | CO | H2O | CO2 | H2 |
0 | 0.200 | 0.300 | 0 | 0 |
2 | 0.138 | 0.238 | 0.062 | 0.062 |
3 | c1 | c2 | c3 | c3 |
4 | c1 | c2 | c3 | c3 |
5 | 0.116 | 0.216 | 0.084 | |
6 | 0.096 | 0.266 | 0.104 |
�ٱ���3��4 minʱ����Ӧ����_______________״̬��c1��ֵ_____________0.08 mol/L(��������������С��������������)��
�ڷ�Ӧ��4��5 minʱ��ƽ�����淽���ƶ������ܵ�ԭ����____________(����ĸ����ͬ)������5��6 minʱ����ֵ�����仯�����ܵ�ԭ����________________��
a������ˮ����b�������¶�
c��ʹ�ô���d����������Ũ��