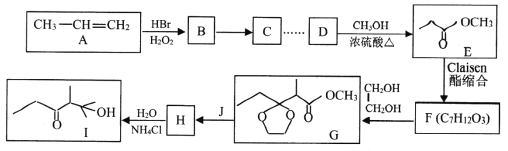
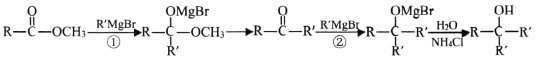
��Ŀ����
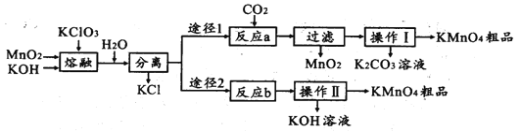
����Ŀ����������(ClO2)����������ˮ�������Դ���Ϊԭ������C1O2�Ĺ�����Ҫ�������ٴ��ξ��ƣ��ڵ������NaC1��Һ����C1O2����ȡ������������ͼ��

�ṩ���Լ�������Na2CO3��Һ������K2CO3��Һ��NaOH��Һ��BaCl2��Һ��Ba(NO3)2��Һ
��1����ʳ��ˮ�к���Ca2+��Mg2+��SO42-�����ʡ����Ӳ���ʱ��������ˮ���ȼ���������Լ�X��ѡ���Լ�X���������Լ������μ�˳������Ϊ��__(�ѧʽ)��
��2�����������У���ʳ��ˮ���ض������µ��õ��������������ᷴӦ����C1O2�����ʱ���ɵ�����B��___�����ʱ������ӦʽΪ__����Ӧ��Ļ�ѧ����ʽΪ___��
��3��ClO2�ܲ��ȶ������������ƣ�������ˮ���յõ�ClO2��Һ��Ϊ�ⶨ������Һ��ClO2�ĺ���������������ʵ�飺
����1��ȷ��ȡClO2��Һ10.00mL��ϡ�ͳ�100mL������
����2����ȡV1mL�������뵽��ƿ�У�����������pH��2.0������������KI���壬ҡ�ȣ��ڰ�������30���ӡ�����֪ClO2+I��+H+ I2+Cl��+H2Oδ��ƽ��
����3���Ե�����Һ��ָʾ������cmol��L-1Na2S2O3��Һ�ζ����յ㣬����Na2S2O3��ҺV2mL��(��֪��I2+2S2O32-=2I-+S4O62-)
��ȷ��ȡ10.00mLClO2��Һ�IJ���������__��
����������3�еζ��յ��������___��
����ʵ����ʹ�õ�Na2S2O3����Һ�������������������ʣ���ʵ����__(�ƫ�ߡ�����ƫ�͡�����Ӱ�족)
�ܸ�����������ɼ����ԭC1O2��Һ��Ũ��Ϊ__mol��L-1(�ú���ĸ�Ĵ���ʽ��ʾ)��
���𰸡�BaCl2��NaOH��Na2CO3(Na2CO3����BaCl2����) H2 C1����6e��+3H2O=ClO3��+6H+ 2NaClO3+4HCl=2ClO2��+2NaCl+2H2O+Cl2�� ��ʽ�ζ��� ��Һ��ɫǡ����ʧ����30s�ڲ��ָ�ԭɫ ƫ�� ![]()
��������
��1���Դ�����Һ�еĸ����ӡ�þ���ӡ���������ӵ��������ӳ�ȥ�Ĺ��̣����ݳ��Ӳ�����������Ϊԭ�����Ȼ�����ȥ��������ӣ���̼���Ƴ�ȥ�����Ӻ����ı����ӣ����������Ƴ�ȥþ���ӣ���Ҫע�����̼������ҺҪ����ټ��룬��������ı���������ȥ���������������Լ��������ȼ�Ҳ���Ժ�ӣ��Գ�ȥ��������������Ӱ�죬��˳������ļ���˳��ΪNaOH��BaCl2��Na2CO3��Ҳ����BaCl2��NaOH��Na2CO3��
����BaCl2��NaOH��Na2CO3(Na2CO3����BaCl2����)��
��2����ʳ��ˮ���ض������µ��õ������ƣ���Ԫ�صĻ��ϼ���-1������Ϊ+5�ۣ�ʧ���ӣ�����������Ӧ���ù����ڵ���������ɣ��缫��ӦʽΪ��C1����6e��+3H2O=ClO3��+6H+�������õ��ӣ�������ԭ��Ӧ��ˮ�������õ��������������ų���������Ӧ��Ϊ���õ��������������ᷢ��������ԭ��Ӧ����C1O2�����ݵ��ӵ�ʧ�غ㣬�����غ㣬��ѧ����ʽΪ��2NaClO3+4HCl=2ClO2��+2NaCl+2H2O+Cl2����
����H2��C1����6e��+3H2O=ClO3��+6H+�� 2NaClO3+4HCl=2ClO2��+2NaCl+2H2O+Cl2����
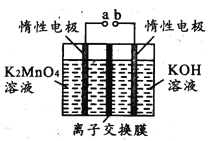
��3���ٽ��еζ�ʵ�飬��Ҫʹ�����ζ��ܣ���ʽ�ζ�������ʢ�����Ի���������Һ����ʽ�ζ��ܿ���Һ��Ļ��������ױ�������һ������ʢ�ż�����Һ��ClO2���������ԣ�Ӧʢ������ʽ�ζ����У�
��Ϊ����ʽ�ζ��ܡ�
���Ե�����Һ��ָʾ������cmol��L-1Na2S2O3��Һ�ζ����յ㣬������֪��ӦI2+2S2O32-=2I-+S4O62-���������һ��Na2S2O3��Һ����ɫǡ����ʧ����30s�ڲ��ָ�ԭɫ��
��Ϊ���������һ��Na2S2O3��Һ����ɫǡ����ʧ����30s�ڲ��ָ�ԭɫ��
����ʵ����ʹ�õ�Na2S2O3����Һ�������������������ʣ�Na2S2O3��Ũ�Ȼή�ͣ��ζ�ʱ���ı�Һ�����������![]() ����ʵ������ƫ�ߣ�
����ʵ������ƫ�ߣ�
��Ϊ��ƫ�ߡ�
����cmol��L-1Na2S2O3��Һ�ζ����յ㣬����Na2S2O3��ҺV2mL �����ݷ�ӦI2+2S2O32-=2I-+S4O62-�� 2ClO2+10I��+8H+=5I2+2Cl��+4H2O��֪��2ClO2~5I2~ 10S2O32-��n��S2O32-��=cV2��10-3mol������V1mLClO2����Һ�к��е�ClO2�����ʵ���Ϊ2cV2��10-4mol����10mL��ԭ��Һ����ClO2�����ʵ���Ϊ��2cV2��10-4mol��![]() ��10-2mol������ԭClO2��Һ�����ʵ���Ũ��Ϊ��
��10-2mol������ԭClO2��Һ�����ʵ���Ũ��Ϊ��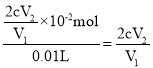 mol/L��
mol/L��
����![]() ��
��

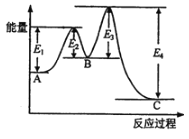
����Ŀ��һ���¶��£�2L�ĺ����ܱ������з�����ӦA(g)+2B(g)3C(g)����Ӧ�����еIJ����������±���ʾ������˵����ȷ���ǣ� ��
n/mol t/min | n(A) | n(B) | n(C) |
0 | 2.0 | 2.4 | 0 |
5 | 0.9 | ||
10 | 1.6 | ||
15 | 1.6 |
A.ƽ��״̬ʱ��c(C)=0.6mol/LB.�÷�Ӧ��10min��Ŵﵽƽ��
C.05min��A��ʾ��ƽ����Ӧ����Ϊ0.06molL-1min-1D.����B��ƽ��ת����Ϊ20%
����Ŀ�������ˮ��Һ�д��ڵ���ƽ�⡢ˮ��ƽ�⡢�ܽ�ƽ�⣬��ش��������⡣
��1����֪��������ĵ��볣�����±���
���� | CH3COOH | HCN | H2CO3 |
���볣��(25��) | Ka = 1.8��10-5 | Ka=4.3��l0-10 | Ka1=5.0��l0-7 Ka2=5.6��l0-11 |
��0.1 moI/L NaCN��Һ��0.1mol/L NaHCO3��Һ�У�c(CN-)______c(HCO3-)(����>������<������=��)��
�ڳ����£����ʵ���Ũ����ͬ��������Һ��A��CH3COONa B��NaCN C��Na2CO3����pH�ɴ�С��˳����________(����)��
�۽�����CO2ͨ��NaCN��Һ����Ӧ�����ӷ���ʽ��__________��
�������£�����Ũ�ȵ�CH3COONa��ҺpH=9�������ӷ���ʽ��ʾ��Һ�ʼ��Ե�ԭ����______����Һ��c(CH3COO-)/c(CH3COOH) =________��
��2��ij�¶���, PH=3��������[OH-]=10-9 mol/L. ���¶���, PH=2��H2SO4��PH=11��NaOH��Ϻ�PH��Ϊ9,���������������Ƶ������Ϊ______.
��3�������£���0.100 mol/L������Һ�ζ�20.00mL 0.l00mol/L ��ij��ˮ��Һ���ζ�������ͼ��ʾ��������Ϊ������������

��d����ʾ����Һ������Ũ���ɴ�С��˳������Ϊ_____��
��b����ʾ����Һ��c(NH4+)��c(NH3��H2O)=______(д��ȷ��ֵ)��
��4����SO2����ˮ�γɵĶ�Ԫ������Һ�У���SԪ�ص�ij��ռ���к�SԪ���������ʵ�����������ҺpH�Ĺ�ϵ����ͼ��ʾ�������Ļ�ѧʽΪ_______���ö�Ԫ�����һ�����볣��ΪKa1����pKa1=-lgKa1��____��