��Ŀ����
��ɫ��������п���׳�𩷯����ҽ������������������ҵ�����������������ɫ���ϣ�п���ף��ȡ�ijʵ��С�����������Һ�Ʊ�����п���壬����������ʵ�飺
��1��ȡ50mL�����Һ�����ˡ���ȥ���������ʺ���ZnO������ҺʹpHԼ����2�����ȡ�������Ũ���Ƶýϸ��¶��µ�����п������Һ����ȴ�ᾧ���õ����Ƶ�����п���塣
�ټ�����������п������ҺʹpH��2Ŀ���� ��
�ڼ���������Ũ����Һʱ��Ӧ���ȵ� ʱ��ֹͣ���ȡ�
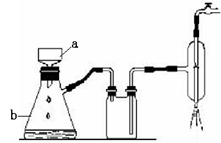
��2���־������ձ�������������ˮ�ܽ⣬�μ�1��2��ϡ���ᣬ�÷�ˮԡ����������ȫ���ܽ⡣ֹͣ���ȣ�������Ȼ��ȴ���ᾧ�����ˣ�װ����ͼ��ʾ���������þ�����������ˮ�Ҵ�ϴ��1-2�Σ��õ��ϴ�������п���塣
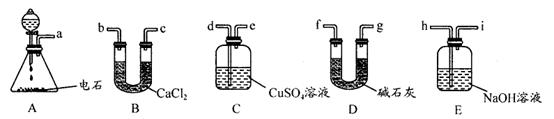
��д���������������ơ�a ��b
�ڳ��˸���ͨ������ȣ����˵õ������ϸ����⣬����һ���ŵ��� ��
����������ˮ�Ҵ�ϴ�Ӿ����Ŀ���� ��
��3������ȡ����ʱ�����п���л�������������ͭ���ʣ�������п������ ����С������ޡ���Ӱ�죬ԭ���� ��
��1��ȡ50mL�����Һ�����ˡ���ȥ���������ʺ���ZnO������ҺʹpHԼ����2�����ȡ�������Ũ���Ƶýϸ��¶��µ�����п������Һ����ȴ�ᾧ���õ����Ƶ�����п���塣
�ټ�����������п������ҺʹpH��2Ŀ���� ��
�ڼ���������Ũ����Һʱ��Ӧ���ȵ� ʱ��ֹͣ���ȡ�
��2���־������ձ�������������ˮ�ܽ⣬�μ�1��2��ϡ���ᣬ�÷�ˮԡ����������ȫ���ܽ⡣ֹͣ���ȣ�������Ȼ��ȴ���ᾧ�����ˣ�װ����ͼ��ʾ���������þ�����������ˮ�Ҵ�ϴ��1-2�Σ��õ��ϴ�������п���塣

��д���������������ơ�a ��b
�ڳ��˸���ͨ������ȣ����˵õ������ϸ����⣬����һ���ŵ��� ��
����������ˮ�Ҵ�ϴ�Ӿ����Ŀ���� ��
��3������ȡ����ʱ�����п���л�������������ͭ���ʣ�������п������ ����С������ޡ���Ӱ�죬ԭ���� ��
��1���ٽ�����ת��������п�����ȥ���ᣩ���������������ʣ�2�֣�
����Һ������־�Ĥ 2��
��2���ٲ���©����1�֣� ����ƿ��1�֣� �ڹ����ٶȿ� ��1�֣�
�������Ҵ��Ļӷ�����ȥ������渽�ŵ�ˮ�� ��2�֣�
��3���ޣ�1�֣�ͭ����ϡ���ᷴӦ�����˳�ȥ������Ȼ��ϡ���ᷴӦ����������������п�������ã�ֻҪп���������Ϳ��û����������������˳�ȥ��2��
����Һ������־�Ĥ 2��
��2���ٲ���©����1�֣� ����ƿ��1�֣� �ڹ����ٶȿ� ��1�֣�
�������Ҵ��Ļӷ�����ȥ������渽�ŵ�ˮ�� ��2�֣�
��3���ޣ�1�֣�ͭ����ϡ���ᷴӦ�����˳�ȥ������Ȼ��ϡ���ᷴӦ����������������п�������ã�ֻҪп���������Ϳ��û����������������˳�ȥ��2��
��1������Һ�к��й��������ᣬ���Լ�����������п������ҺʹpH��2Ŀ���ǽ�����ת��������п���������������ʡ�
������ʱ����Һ������־�Ĥʱ������ֹͣ���ȡ�
��2���ٸ���װ���ص���жϣ�a�Dz���©����b������ƿ��
�ڲ���©�����ŵ����ͨ©����ȹ����ٶȿ졣
���Ҵ���ˮ�ǻ��ܵģ�������������ˮ�Ҵ�ϴ�Ӿ����Ŀ���ǣ������Ҵ��Ļӷ�����ȥ������渽�ŵ�ˮ�֡�
��3����Ϊͭ����ϡ���ᷴӦ�����˼��ɳ�ȥ������Ȼ��ϡ���ᷴӦ����������������п�������ã�ֻҪп���������Ϳ��û����������������˳�ȥ�����Բ���Ӱ������п��������
������ʱ����Һ������־�Ĥʱ������ֹͣ���ȡ�
��2���ٸ���װ���ص���жϣ�a�Dz���©����b������ƿ��
�ڲ���©�����ŵ����ͨ©����ȹ����ٶȿ졣
���Ҵ���ˮ�ǻ��ܵģ�������������ˮ�Ҵ�ϴ�Ӿ����Ŀ���ǣ������Ҵ��Ļӷ�����ȥ������渽�ŵ�ˮ�֡�
��3����Ϊͭ����ϡ���ᷴӦ�����˼��ɳ�ȥ������Ȼ��ϡ���ᷴӦ����������������п�������ã�ֻҪп���������Ϳ��û����������������˳�ȥ�����Բ���Ӱ������п��������

��ϰ��ϵ�д�
 ���ٴ�����ɽ����ϵ�д�
���ٴ�����ɽ����ϵ�д�
�����Ŀ
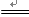 CaSO4 + H2O + CO2��
CaSO4 + H2O + CO2�� MnCl2 + Cl2��+ 2H2O
MnCl2 + Cl2��+ 2H2O