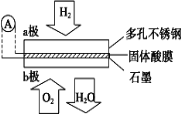
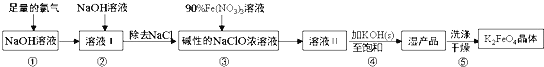
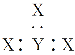��Ŀ����
����Ŀ����ˮ�Ȼ������л��ϳɹ�ҵ��һ����Ҫ�Ĵ�������ҵ����Al2O3�Ʊ���ˮ�Ȼ����ķ�ӦΪ2Al2O3��6Cl2![]() 4AlCl3��3O2��
4AlCl3��3O2��
(1)ʵ���������������ӷ���ʽΪ_________________________��������ˮ���ɵĴ�����ĵ���ʽΪ__________________________
(2)AlCl3��������ˮ������ԭ����(�����ӷ���ʽ��ʾ) ___________________________
(3)Al��Mg�Ͻ�ǰ��NaOH��Һ����Al2O3Ĥ�������ӷ���ʽΪ________________
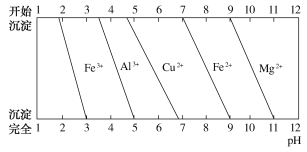
(4)Ϊ����ij���Ͻ�ijɷ֣�������ϡ���Ὣ����ȫ�ܽ����NaOH��Һ��pH����pH��3.4ʱ��ʼ���ֳ������ֱ���pHΪ5��9ʱ���˳����������μ�NaOH��Һ�������ɡ����ͼ����Ϣ�ƶϸúϽ��г�������еĽ�����___________________________��
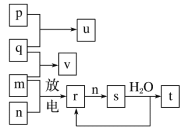
���𰸡�MnO2��4H����2Cl��![]() Mn2����Cl2����2H2O
Mn2����Cl2����2H2O ![]() Al3����3H2O===Al(OH)3��3H�� Al2O3��2OH��===2AlO2-��H2O Cu
Al3����3H2O===Al(OH)3��3H�� Al2O3��2OH��===2AlO2-��H2O Cu
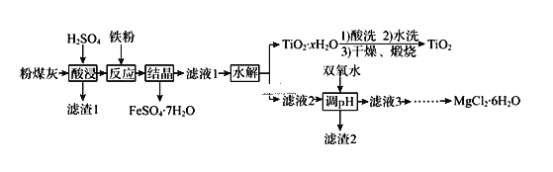
��������
�������ǿ�����ԣ�����ϡ�����ܽ�������������������Ͻ������ӿ�ʼ��������ȫ����ʱ��pH�������Ͻ�ijɷ֡�
(1)ʵ���ҳ��ö���������Ũ���Ṳ�������������ӷ���ʽΪMnO2��4H����2Cl��![]() Mn2����Cl2����2H2O����������ˮ��������ʹ����ᣬ������ĵ���ʽΪ
Mn2����Cl2����2H2O����������ˮ��������ʹ����ᣬ������ĵ���ʽΪ![]() ��
��
(2)AlCl3ˮ�����������������壬����ˮ���������ʣ��ʿ�����ˮ�������ӷ���ʽAl3����3H2O��Al(OH)3�����壩��3H����
(3)Al2O3���������������NaOH��Һ����Al2O3Ĥ�����ӷ���ʽΪAl2O3��2OH��===2AlO2-��H2O��
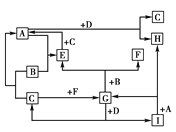
(4)���Ͻ���������ϡ�����Al��Al3+��Cu��Cu2+��Mg��Mg2+��Fe��Fe3+��ϡ��������ʱ��������Fe2+������NaOH��Һ��pH����pH��3.4ʱ�ſ�ʼ���ֳ���������Һ����Fe3+���Ͻ���������pH=5ʱAl3+��ȫ������������������Cu2+������pH=9ʱ����ֻ��Cu(OH)2����Ͻ�����ͭ�������μ�NaOH��Һ�������ɣ���Ͻ�����þ���ʸúϽ�Ϊ��ͭ�Ͻ�