��Ŀ����
7������˵����ȷ���ǣ�������| A�� | ����ģ��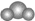 �����Ա�ʾ������̼���ӣ�Ҳ���Ա�ʾˮ���� �����Ա�ʾ������̼���ӣ�Ҳ���Ա�ʾˮ���� | |
| B�� | �����£���0.4mol/L HA��Һ��0.2mol/LNaOH��Һ�������ϣ����Ի��ʱ��Һ����ı仯����û����Һ��pH=5��������Һ����ˮ�������c��H+��=1��10-5mol/L | |
| C�� | ��̼�²��ϡ�̼������ĭ����ÿ����ĭ����Լ4000��̼ԭ�ӣ�ֱ��Լ6��9nm���ڵ���-183��ʱ����ĭ�������ô��ԣ���̼������ĭ����ʯī��Ϊͬλ�� | |
| D�� | ��֪ Ag2CrO4��KspΪ1.12��10-12���������1��10-4 mol•L-1��AgNO3��Һ��1��10-4 mol•L-1��K2Cr04��Һ��ϣ�������Ag2CrO4�������� |
���� A��������̼��ֱ���ͷ��ӣ�ˮ������V�ͷ��ӣ�
B�������£���0.4mol/L HA��Һ��0.2mol/LNaOH��Һ�������ϣ����Һ��HA��NaA��Ũ����ȣ������Һ��pH=5����Һ��ʾ���ԣ���Һ������������Ϊˮ����ģ�
C����̼������ĭ������̼������ʯī��Ϊͬ�������壻
D����һ���¶��£�Ksp��һ��������ͨ���Ƚ�Ksp����Һ���й�����Ũ���ݵij˻�--���ӻ�Qc����Դ�С�������ж����ܵ�����ڸ��������³����ܷ����ɻ����ܽ⣬��Qc��Kspʱû�г���������
��� �⣺A��������̼��ֱ���ͷ��ӣ�ˮ������V�ͷ��ӣ��ñ���ģ����ˮ����ģ�ͣ���A����
B����û����Һ��pH=5��HA��������Һ��ʾ���ԣ���Ӧ��Ļ��Һ��������������ˮ����ģ�����ˮ�����������Ũ��Ϊ��c��H+��=1��10-9mol/L����B����
C����̼������ĭ������̼������ʯī��Ϊͬ�������壬ͬλ����ԭ�ӣ���C����
D���������Ϻ�c��Ag+��=5��10-5mol/L��c��CrO42-��=5��10-5mol/L��Qc=c2��Ag+����c��CrO42-��=��5��10-5��2��5��10-5��Ksp��Ag2CrO4��=9.0��10-12����û�г�����������D��ȷ��
��ѡD��
���� ������Ҫ�����˷��ӽṹ�ı���ģ�ͣ���Һ����Ũ�ȵļ��㣬ͬλ�ص��ж��Լ������ܽ�ƽ����жϵȣ��ѶȲ���ע�����֪ʶ�Ļ��ۣ�

| A�� | ȼ�շ�Ӧ���Ƿ��ȷ�Ӧ | |
| B�� | ���ڿ��淴Ӧ��aA��g��+bB��g��?bC��g��+dD��g�����������Ӧ���ȣ��淴Ӧһ������ | |
| C�� | ����ȼ������ˮ��һ�����ȵĻ�ѧ��Ӧ��˵��1mol��H2����������1mol��H2O������ | |
| D�� | ʯīת��Ϊ���ʯ��Ҫ��������������ʯī�Ļ�ѧ���ʸ��ȶ� |
| A�� | Ư�۾� | B�� | ��ʯ�� | C�� | �������� | D�� | �������� |
| A�� | ��������Ҳ������Cu | |
| B�� | ����ÿ����3molH2������1mol Cr2O72-����ԭ | |
| C�� | һ��ʱ�����Һ�з�����ӦCr2O72-+6Fe2++14H+�T2Cr3++6Fe3++7H2O | |
| D�� | ���������Ҫ�Ӽʹ��Һ�е�������ת��Ϊ���� |
| A�� | Z��X��Y | B�� | X��Y��Z | C�� | Z��Y��X | D�� | X��Z��Y |

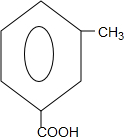 +
+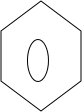 $\stackrel{PCl_{3}}{��}$
$\stackrel{PCl_{3}}{��}$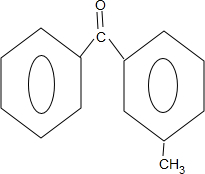 +H2O��
+H2O��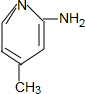 ��
��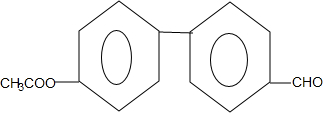 ��ֻдһ�֣���
��ֻдһ�֣���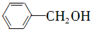 ��
��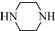 Ϊԭ���Ʊ�
Ϊԭ���Ʊ�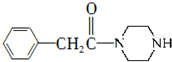 �ĺϳ�·������ͼ�����Լ����ã���
�ĺϳ�·������ͼ�����Լ����ã���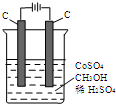 ��ⷨ�������״���ˮ����ɵ���Ⱦ��ԭ���ǣ�ͨ�罫Co2+������Co3+��Ȼ��Co3+���״�������CO2��H+����ʯīϩ������ȥCo2+����������ͼװ��ģ���������̣���
��ⷨ�������״���ˮ����ɵ���Ⱦ��ԭ���ǣ�ͨ�罫Co2+������Co3+��Ȼ��Co3+���״�������CO2��H+����ʯīϩ������ȥCo2+����������ͼװ��ģ���������̣���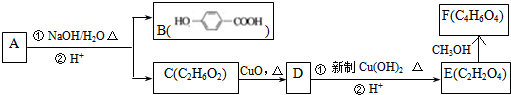
 �Ļ�ѧ�Լ���NaHCO3��д��ѧʽ����
�Ļ�ѧ�Լ���NaHCO3��д��ѧʽ����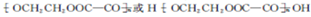 ��Eת��ΪF�Ļ�ѧ��Ӧ������������Ӧ��ȡ����Ӧ��
��Eת��ΪF�Ļ�ѧ��Ӧ������������Ӧ��ȡ����Ӧ��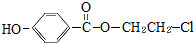 +3NaOH$\stackrel{��}{��}$HOCH2CH2OH+NaCl+
+3NaOH$\stackrel{��}{��}$HOCH2CH2OH+NaCl+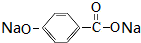 +H2O��
+H2O�� ��
��