��Ŀ����
��14�֣���������Ҫ������Դ��������Ϊ�������õĹؼ��������ǵ�ǰ��ע���ȵ�֮һ��
��1�������������Դ����ȼ�ղ���Ϊ__________��
��2��NaBH4��һ����Ҫ�Ĵ������壬����ˮ��Ӧ�ﵽNaBO2���ҷ�Ӧǰ��B�Ļ��ϼ۲��䣬�÷�Ӧ�Ļ�ѧ����ʽΪ___________����Ӧ����1mol NaBH4ʱת�Ƶĵ�����ĿΪ__________��
��3������ɽ����л�������û�����ͱ�֮��Ŀ��淴Ӧ��ʵ������ͼ��⣺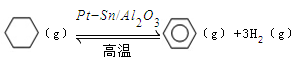 ��ij�¶��£�������ܱ������м��뻷���飬��ʼŨ��Ϊa mol/L��ƽ��ʱ����Ũ��Ϊbmol/L���÷�Ӧ��ƽ�ⳣ��K��_____��
��ij�¶��£�������ܱ������м��뻷���飬��ʼŨ��Ϊa mol/L��ƽ��ʱ����Ũ��Ϊbmol/L���÷�Ӧ��ƽ�ⳣ��K��_____��
��4��һ�������£���11ͼʾװ�ÿ�ʵ���л���ĵ绯ѧ���⣨���������л����
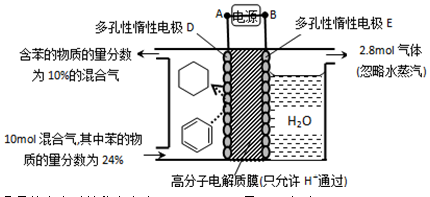
�ٵ����е����ƶ�����Ϊ____________��
������Ŀ�����ĵ缫��ӦʽΪ_________��
�۸ô���װ�õĵ���Ч�� ��_____��
��_____��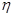 ��
�� ��100%������������С�����1λ��
��100%������������С�����1λ��
��1�������������Դ����ȼ�ղ���Ϊ__________��
��2��NaBH4��һ����Ҫ�Ĵ������壬����ˮ��Ӧ�ﵽNaBO2���ҷ�Ӧǰ��B�Ļ��ϼ۲��䣬�÷�Ӧ�Ļ�ѧ����ʽΪ___________����Ӧ����1mol NaBH4ʱת�Ƶĵ�����ĿΪ__________��
��3������ɽ����л�������û�����ͱ�֮��Ŀ��淴Ӧ��ʵ������ͼ��⣺
 ��ij�¶��£�������ܱ������м��뻷���飬��ʼŨ��Ϊa mol/L��ƽ��ʱ����Ũ��Ϊbmol/L���÷�Ӧ��ƽ�ⳣ��K��_____��
��ij�¶��£�������ܱ������м��뻷���飬��ʼŨ��Ϊa mol/L��ƽ��ʱ����Ũ��Ϊbmol/L���÷�Ӧ��ƽ�ⳣ��K��_____����4��һ�������£���11ͼʾװ�ÿ�ʵ���л���ĵ绯ѧ���⣨���������л����

�ٵ����е����ƶ�����Ϊ____________��
������Ŀ�����ĵ缫��ӦʽΪ_________��
�۸ô���װ�õĵ���Ч��
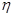 ��_____��
��_____��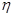 ��
�� ��100%������������С�����1λ��
��100%������������С�����1λ����1��ˮ��H2O ��2��NaBH4��2H2O=NaBO2��4H2����4NA��2.408��1024
��3��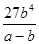 mol3/L3 ��4����A��D ��C6H6��6H����6e����C6H12 ��64.3%
mol3/L3 ��4����A��D ��C6H6��6H����6e����C6H12 ��64.3%
��3��
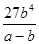 mol3/L3 ��4����A��D ��C6H6��6H����6e����C6H12 ��64.3%
mol3/L3 ��4����A��D ��C6H6��6H����6e����C6H12 ��64.3%�����������1��������ȼ�ղ�����ˮ��
��2����Ӧǰ��BԪ�صĻ��ϼ۲��䣬��Ӧǰ��BԪ�صĻ��ϼ۾��ǣ�3�ۣ���˷�ӦǰNaBH4����Ԫ�صĻ��ϼ��ǣ�1�ۡ�ˮ����Ԫ�صĻ��ϼ��ǣ�1�ۣ���˷�Ӧ�л����������ɣ���Ӧ�Ļ�ѧ����ʽΪNaBH4��2H2O=NaBO2��4H2����NaBH4����Ԫ�صĻ��ϼ۴ӣ�1�����ߵ�0�ۣ����1molNaBH4�ڷ�Ӧ��ʧȥ4mol���ӣ�����Ŀ��4NA��2.408��1024��
��3��ƽ��ʱ����Ũ����b mol/L������ݷ�Ӧ�ķ���ʽ��֪���Ļ������Ũ����b mol/L������������Ũ����3 b mol/L����ƽ��ʱ�������Ũ��Ϊ��a��b��mol/L�����ڻ�ѧƽ�ⳣ������һ�������£������淴Ӧ�ﵽƽ��״̬ʱ��������Ũ�ȵ���֮���ͷ�Ӧ��Ũ�ȵ���֮���ı�ֵ������¶��·�Ӧ��ƽ�ⳣ��Ϊ
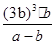 ��
��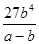 mol3/L3��
mol3/L3����4���ٱ����ɻ��������ڵ��ⷴӦ������ǻ�ԭ��Ӧ�����缫D���������缫E����������˵����е��ӵ�����������A��D��
�ڱ��õ��������ɻ�������Ŀ�������ڴ������ӽ���Ĥ�������������������ƶ�����缫��ӦʽΪC6H6��6H����6e����C6H12��
����������2.8mol���壬������Ӧ��������OH���ŵ����ɵ���������ת�Ƶ��ӵ����ʵ�����2.8mol��4��11.2mol�����������ı������ʵ�����xmol����ͬʱ���� xmol�����飬���ݵ缫��ӦʽC6H6��6H����6e����C6H12��֪�õ�������6xmol�����ݵ����غ��֪����������������
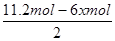 ��5.6mol��3xmol������
��5.6mol��3xmol������ ��0.1�����x��1.2����˴���װ�õĵ���Ч�ʣ�
��0.1�����x��1.2����˴���װ�õĵ���Ч�ʣ�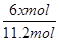 ��100%��64.3%��
��100%��64.3%��
��ϰ��ϵ�д�
 ����ѧ���ʱѧ����ϵ�д�
����ѧ���ʱѧ����ϵ�д�
�����Ŀ
 Fe(OH)2+2H+���ڴ���Һ�м���ϡ���ᣬ����Һ����ɫ�仯Ϊ( )
Fe(OH)2+2H+���ڴ���Һ�м���ϡ���ᣬ����Һ����ɫ�仯Ϊ( ) 4NO+6H2O(g)��������������ȷ���� �� ��
4NO+6H2O(g)��������������ȷ���� �� ��  2C(��)�ﵽƽ��ı�־��
2C(��)�ﵽƽ��ı�־�� 2SO3��g����һ�����������ܱ������з�Ӧ���ﵽƽ��״̬�ı�־�ǣ� ��
2SO3��g����һ�����������ܱ������з�Ӧ���ﵽƽ��״̬�ı�־�ǣ� ��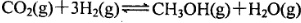 ��5 min��Ӧ�ﵽƽ��ʱc(CH3OH)Ϊ0.2 mol
��5 min��Ӧ�ﵽƽ��ʱc(CH3OH)Ϊ0.2 mol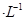 ��CO2(g)��ƽ�����ʵ���Ũ��c(CO2)���¶ȹ�ϵ��ͼ��ʾ������˵���������
��CO2(g)��ƽ�����ʵ���Ũ��c(CO2)���¶ȹ�ϵ��ͼ��ʾ������˵���������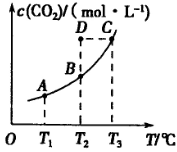
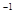
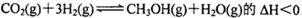
 I3��(aq)���ڷ�Ӧ��ƽ����ϵ�У�c(I3��)���¶�T�Ĺ�ϵ��ͼ��ʾ�������ϵ��κ�һ�㶼��ʾƽ��״̬����
I3��(aq)���ڷ�Ӧ��ƽ����ϵ�У�c(I3��)���¶�T�Ĺ�ϵ��ͼ��ʾ�������ϵ��κ�һ�㶼��ʾƽ��״̬����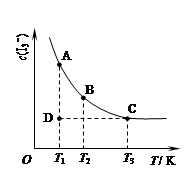
 2SO3(g)��ij�¶��£���2mol SO2��1mol O2����10L�ܱ������У���Ӧ��ƽ���SO2��ƽ��ת����(��)����ϵ��ѹǿ(p)�Ĺ�ϵ��ͼ����ʾ��������˵����ȷ����
2SO3(g)��ij�¶��£���2mol SO2��1mol O2����10L�ܱ������У���Ӧ��ƽ���SO2��ƽ��ת����(��)����ϵ��ѹǿ(p)�Ĺ�ϵ��ͼ����ʾ��������˵����ȷ����