��Ŀ����
13�����⣨MoS2�����������豸��������ȹ����ϩ����C60���������ƣ��ش��������⣺��1����λ�ڵ�������VIB�壮�������ڵ���һ����ͬ��Ԫ�صķ���ΪCr����Ԫ�ػ�̬ԭ�ӵĵ�������Ų����ܼ�Ϊ3d�����ܼ����ܵĵ�����Ϊ5��
��2��̼�����й����������
| Ԫ�� | ԭ�Ӱ뾶 | �縺�� | ��һ������ |
| C | 67pm | 2.55 | 1125.8kJ/mol |
| S | 88pm | 2.58 | 1036.7kJ/mol |
��д����֤���ý��۵Ļ�ѧ����ʽH2SO4+Na2CO3=Na2SO4+CO2��+H2O��
��3���Ӳ�ͬ�Ƕȹ۲�MoS2�ľ���ṹ��ͼ1��2������MoS2�ľ���ṹ�ش�
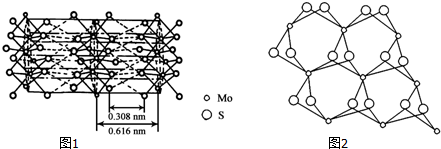
�������λ��Ϊ6��
��Mo��S֮��Ļ�ѧ��ΪAC������ţ���
A�����Լ� B���Ǽ��Լ� C����λ�� D�������� E�����»���
�ۻ����������ӵ������ܷdz����죬��ԭ����MoS2���в�״�ṹ��Mo��Sͬ����Թ��ۼ���ϣ������֮��ͨ�����»�����ϣ��������ò����������Ի�����
��4������̼�ڸ����·�Ӧ�ɵõ����ɰ��SiC������ṹ����ʯ�ṹ���ƣ��������ʯ������һ���Cԭ�ӻ���Siԭ����ͬ��ԭ�Ӳ��ɼ�����õĽ��ɰ��SiC���ṹ�����������һ����ԭ��Ϊ���ģ���SiC�����й�ԭ�����������̼ԭ�ӵ��������Ϊd�������ԭ�Ӵν��ĵڶ�����12��ԭ�ӣ���������ԭ�ӵľ�����$\frac{2\sqrt{6}}{3}$d��
���� ��1����λ�ڵ�������VIB�壬�������ڵ���һ����ͬ��Ԫ�ؼ���������VIB�壬Ϊ��Ԫ�أ�����ΪCr�������������ԭ�����������������4s�������3d��������ڰ����ṹ��һ���ȶ��ṹ��������4s�������1�����ӣ�3d�������5�����ӣ����ǰ����ṹ����һ���ȶ��ṹ��
��2��Ԫ�صķǽ�����Խǿ���縺��Խ���ݱ��е����ݿ��жϣ����Ը�����ۺ����������ǿ������֤����
��3���ٸ����ұ�ͼ�ж�ÿ��Moԭ����Χ���������Sԭ����Ŀ��
���ṩ�չ�����ṩ�µ��ӶԵ�ԭ�Ӽ����γ���λ������λ�����ڹ��ۼ���
��MoS2�ṹ��ʯī���ƣ�����ʯī�ṹ������
��4����������Si����ĵ�һ���4��Cԭ�ӣ�ÿһ��������������3��Siԭ�ӣ���12��Siԭ��ƽ���ֲ���һ�������ϣ�����ֱ�������μ�����������ı߳���
��� �⣺��1����λ�ڵ�������VIB�壬�������ڵ���һ����ͬ��Ԫ�ؼ���������VIB�壬Ϊ��Ԫ�أ�����ΪCr�������������ԭ�����������������4s�������3d��������ڰ����ṹ��һ���ȶ��ṹ��������4s�������1�����ӣ�3d�������5�����ӣ����ǰ����ṹ����һ���ȶ��ṹ��
�ʴ�Ϊ��Cr��3d��5��
��2����Ԫ�صķǽ�����Խǿ���縺��Խ���ݱ��е����ݿ�֪��̼�ĵ縺��С��������̼�ķǽ�����������
�ʴ�Ϊ������
�ڿ��Ը�����ۺ����������ǿ������֤���ǽ����Ե�ǿ����������֤���ý��۵Ļ�ѧ����ʽΪH2SO4+Na2CO3=Na2SO4+CO2��+H2O��
�ʴ�Ϊ��H2SO4+Na2CO3=Na2SO4+CO2��+H2O��
��3���ٸ����ұ�ͼ֪��ÿ��Moԭ����Χ���������Sԭ����Ŀ��6��
�ʴ�Ϊ��6��
���ṩ�չ�����ṩ�µ��ӶԵ�ԭ�Ӽ����γ���λ������λ�����ڹ��ۼ�����ͬԪ��֮�����γɵ���λ�����ڼ��Թ��ۼ���
��ѡAC��
��MoS2�ṹ��ʯī���ƣ�����ͼƬ֪��MoS2���в�״�ṹ��Mo��Sͬ����Թ��ۼ���ϣ������֮��ͨ�����»�����ϣ��������ò����������Ի�����
�ʴ�Ϊ��MoS2���в�״�ṹ��Mo��Sͬ����Թ��ۼ���ϣ������֮��ͨ�����»�����ϣ��������ò����������Ի�����
��4����������Si����ĵ�һ���4��Cԭ�ӣ�ÿһ��������������3��Siԭ�ӣ���12��Siԭ��ƽ���ֲ���һ�������ϣ�F��Cԭ�ӣ�A��B��C��D�ֱ����һ��Siԭ�ӣ�AB��AC��AD��BC��BD��CD�ı߳���ȣ�AF��BF�ij����Ϊd��Fλ�������ϣ�Oλ����������BCD�������ϣ�����������BCD�У�BEΪ������BCD�ĸߣ���CEΪBC��һ�룬��ͼ ������������ı߳�Ϊx��CE�ij�Ϊ0.5x��BE=$\sqrt{x{\;}^{2}-��0.5x��{\;}^{2}}$=$\frac{\sqrt{3}}{2}$x��BO��OE�ij�֮��Ϊ2��1����BO�ij�Ϊ $\frac{\sqrt{3}}{2}$x��$\frac{2}{3}$=$\frac{\sqrt{3}}{3}$x����������ABO�У�AO�ij�=$\sqrt{{x}^{2}-��{\frac{\sqrt{3}}{3}x��}^{2}}$=$\frac{\sqrt{6}}{3}x$����������BFO�У�OF�ij�=$\frac{\sqrt{6}}{3}$x-d=$\sqrt{{d}^{2}-��\frac{\sqrt{3}}{3}x��^{2}}$��x=$\frac{2\sqrt{6}}{3}$d��
������������ı߳�Ϊx��CE�ij�Ϊ0.5x��BE=$\sqrt{x{\;}^{2}-��0.5x��{\;}^{2}}$=$\frac{\sqrt{3}}{2}$x��BO��OE�ij�֮��Ϊ2��1����BO�ij�Ϊ $\frac{\sqrt{3}}{2}$x��$\frac{2}{3}$=$\frac{\sqrt{3}}{3}$x����������ABO�У�AO�ij�=$\sqrt{{x}^{2}-��{\frac{\sqrt{3}}{3}x��}^{2}}$=$\frac{\sqrt{6}}{3}x$����������BFO�У�OF�ij�=$\frac{\sqrt{6}}{3}$x-d=$\sqrt{{d}^{2}-��\frac{\sqrt{3}}{3}x��^{2}}$��x=$\frac{2\sqrt{6}}{3}$d��
�ʴ�Ϊ��12�� $\frac{2\sqrt{6}}{3}$d��
���� ���⿼����ۺϣ��漰֪ʶ��϶࣬���ݼ۵��ӶԻ������ۡ�����ԭ�����縺����ǽ�����ǿ��֮��Ĺ�ϵ�������ļ��㷽����Ԫ�������ɵ����������ע�����֪ʶǨ�Ƶķ������������⣬�ѶȽϴ�

| A�� | ��FeCl3��Һ��ʴӡˢ��·����ͭ����Fe3++Cu�TFe2++Cu2+ | |
| B�� | AlCl3��Һ�м��������İ�ˮ��Al3++3OH-�TAl��OH��3�� | |
| C�� | ��С�մ�����θ����ࣺCO${\;}_{3}^{2-}$+2H+�TCO2��+H2O | |
| D�� | ƫ��������Һ��ͨ�����CO2��AlO${\;}_{2}^{-}$+CO2+2H2O�TAl��OH��3��+HCO${\;}_{3}^{-}$ |
| A�� | �Ƹ���Һ���� | B�� | �Ĵ��ζ� | C�� | ˮ��Һ��Ϊ��ɫ | D�� | �ۻ���С�� |
| A�� | ����ȼ�ϵ���ڼ��Խ����и����ķ�Ӧʽ�ǣ�O2+2H2O+4e-�T4OH- | |
| B�� | NaHCO3��ˮ��Һ�е�ˮ�����ӷ���ʽ��HCO${\;}_{3}^{-}$+H2O�TCO2��+OH- | |
| C�� | ��FeSΪ��������ȥ��ˮ�е�Hg2+��FeS��s��+Hg2+��aq���THgS��s��+Fe2+��aq�� | |
| D�� | ��Na2CO3��ˮ��Һ�������ɣ�����CO2�ӷ������յõ�NaOH���� |
��ش��������⣺
��1����PM2.5����������ˮ�����Ƴɴ�������������ø���������ˮ���������ӵĻ�ѧ��ּ���ƽ��Ũ�������
| ���� | K+ | Na+ | NH4+ | SO42- | NO3- | Cl- |
| Ũ��/mol•L-1 | 4��10-6 | 6��10-6 | 2��10-5 | 4��10-5 | 3��10-5 | 2��10-5 |
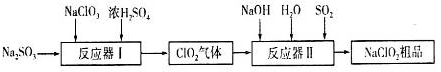
��2��Ϊ����SO2���ŷţ�����ȡ�Ĵ�ʩ�У�
�ٽ�úת��Ϊ�������ȼ�ϣ���֪��
2H2��g��+O2��g���T2H2O��g�� K1
2C��s��+O2��g���T2CO��g�� K2
C��s��+H2O��g���TCO��g��+H2��g�� K=$\sqrt{\frac{{K}_{2}}{{K}_{1}}}$���ú�K1��K2��ʽ�ӱ�ʾ����
��ϴ�Ӻ�SO2���������������ʿ���ϴ�Ӽ�����AB��
A��Ca��OH��2 B��Na2CO3 C��CaCl2 D��NaHSO3
��3������β������NOx��CO�����ɼ�ת��Ϊ��
����֪����������NO�ķ�ӦΪ��N2��g��+O2��g��?2NO��g����H��0����1mol������0.8molN2��0.2molO2��1300��ʱ���ܱ������ڷ�Ӧ�ﵽƽ�⣬���NOΪ8��10-4 mol��������¶��µ�ƽ�ⳣ��K=4��10-6��
������ȼ�Ͳ���ȫȼ��ʱ����CO���������밴���з�Ӧ��ȥCO��2CO��g���T2C��s��+O2��g������֪�÷�Ӧ�ġ�H��0�������������ܷ�ʵ�ֵ����ݣ���H��0����S��0������ϵ�ġ�G=��H-T•��S��0�����Ը����벻��ʵ�֣�
��Ŀǰ��������β��ϵͳ��װ�ô�ת�����ɼ���CO��NO����Ⱦ���仯ѧ��Ӧ����ʽΪ2CO+2NO=2CO2+N2��
�������������ϵ�и����ʵ�Ũ�������
| ��ϵ | c��SO2�� mol/L | c��O2�� mol/L | c��SO3�� mol/L | ��Ӧ���� |
| ��1�� | 0.0600 | 0.400 | 2.000 | |
| ��2�� | 0.0960 | 0.300 | 0.500 | |
| ��3�� | 0.0862 | 0.263 | 1.020 |
��ϵ��1���淽����У�
��ϵ��2����������У�
��ϵ��3����Ӧ�Ѵ�ƽ��״̬��
 ����
����
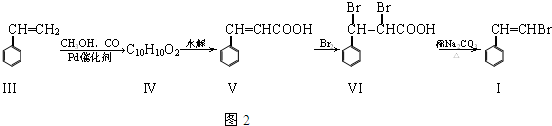
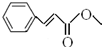 ��
�� ��
�� ��
��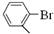 ���뻯���
���뻯��� ��Ҳ�ܷ������Ʒ�Ӧ�ٵ�ż����Ӧ��д������Ľṹ��ʽ
��Ҳ�ܷ������Ʒ�Ӧ�ٵ�ż����Ӧ��д������Ľṹ��ʽ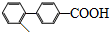 ��
��