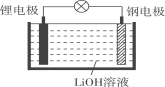
��Ŀ����
����Ŀ�������й�ͼ���˵����ȷ����
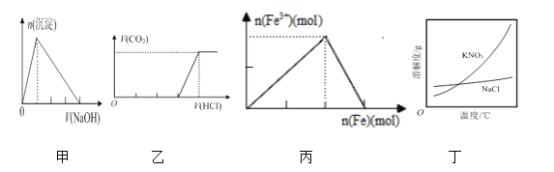
A. ͼ�ױ�ʾ����ij������Һ�еμӹ�����NaOH��Һ�����ɳ��������ʵ�����μ�NaOH��Һ����Ĺ�ϵ
B. ͼ�ұ�ʾ�������ʵ�����NaOH��Na2CO3�Ļ����Һ�еμӹ�����ϡ���ᣬ��������������μ�HCl��Һ����Ĺ�ϵ
C. ͼ����ʾ����ϡ������Һ�м�����������ۣ���Һ��Fe3+���ʵ���������������ʵ����ı仯��ϵ
D. ����ͼ������ȥ����KNO3��������NaCl���á�����Ũ�������ȹ��ˡ��ķ���
���𰸡�C
��������
A. ��ij������Һ�еμ�NaOH��Һֱ����������Һ�������ɳ�����Ȼ����������ܽ⣬ǰ��������û�����ĵ�����������Һ�����֮��Ϊ3:1��ͼ����֮������A������
B. ����NaOH��Na2CO3�ֱ�Ϊ1mol������1molNaOH��Na2CO3�Ļ����Һ�еμӹ�����ϡ���ᣬ���������������ᷴӦ����1molHCl��̼���������ᷴӦ����1molNaHCO3������HCl 1mol�����1molNaHCO3�������ᷴӦ����������̼���壬������HCl 1mol�����Բ�������ǰ��������������֮��Ϊ2:1��ͼ����֮������B������
C. ��ϡ������Һ�м������ۣ��ȷ���Fe+2NO3-+4H+=Fe3++2NO��+2H2O��������������֮�������ӵ����ﵽ���ֵ�������������ۺ�����������������Ӧ��Fe+2Fe3����3Fe2���������ӵ�����Сֱ��Ϊ0������������Ӧ����������Ϊ1:0.5=2:1����ͼ������ϣ�C��ȷ��
D. KNO3�ܽ�����¶ȱ仯�ϴ��Ȼ����ܽ�����¶ȱ仯������˳�ȥ����KNO3��������NaCl���á�����Ũ������ȴ�ᾧ�����ˡ��ķ������з��룬D������
��������������ѡC��

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�����Ŀ��Ϊ���������ܼ��š��͡���̼���á���Ŀǰ��ҵ����һ�ַ�������CO2������ȼ���Ҵ���һ�������·�����Ӧ��2CO2(g)��6H2(g)![]() CH3CH2OH(g)��3H2O(g)����H<0��
CH3CH2OH(g)��3H2O(g)����H<0��
��1����һ�������£���20 L�ܱ������а����ʵ�����Ϊ1��3����CO2��H2���¶���450 K��n(H2)��ʱ��仯�����ʾ��
t/min | 0 | 1 | 3 | 5 |
n(H2)/mol | 8 | 6 | 5 | 5 |
��450 �桢0��1 min��v(CH3CH2OH)��________�����¶��¸÷�Ӧ�Ļ�ѧƽ�ⳣ��Ϊ________(���������λ��Ч����)��
��2����5 MPa�²��ƽ����ϵ�и����ʵ�����������¶ȵı仯������ͼ��ʾ��

�����ұ�ʾ���� ________(�����ʵĻ�ѧʽ)�����������ͼ����A���Ӧ���������b��________%(���������λ��Ч����)��
��3�����д�ʩ����ʹ��ѧƽ��������Ӧ�����ƶ�����________��
A�������¶�
B����CH3CH2OH(g)��ʱҺ�����
C��ѡ���Ч����
D���ٳ���l mol CO2��3 mol H2
��4��25 �桢1.01��105Paʱ��9.2 gҺ̬�Ҵ���ȫȼ�գ����ָ���ԭ״̬ʱ���ų�273.4 kJ��������д����ʾ�Ҵ�ȼ�յ��Ȼ�ѧ����ʽ��________________________��
��5����ʯīΪ�缫���������ơ��Ҵ���ˮ������Ϊԭ�ϣ������Ƴ��Ҵ���ȼ�ϵ�أ�д��������ԭ��Ӧ�ĵ缫��Ӧʽ��_____________________________________��