��Ŀ����
A��B��C��D���ֶ�����Ԫ�أ�A��B��Cͬ���ڣ�A��ԭ�Ӱ뾶��ͬ���������ģ�B��Dͬ���塣��֪DԪ�ص�һ�ֵ������ճ���������ˮ�����õ���������CԪ�صĵ��ʿ��Դ�A��B��Ԫ����ɵĻ������ˮ��Һ���û���BԪ�صĵ��ʡ�
��1��CԪ�������ڱ��е�λ�� ��
��2��AԪ����ˮ��Ӧ�����ӷ���ʽ�� ��
��3��д��CԪ�صĵ��ʴ�A��B��Ԫ����ɵĻ������ˮ��Һ���û���BԪ�صĵ��ʵĻ�ѧ����ʽ ��
��4����
������Ư���ԣ����ߵ�Ư��ԭ�� �������ͬ����ͬ����
��5�������Ǻϳɰ�����Ҫԭ�ϣ��ϳɰ���Ӧ���Ȼ�ѧ����ʽ���£�
3H2��N2 2NH3 ��H=��92��4kJ��mol��1
�ٵ��ϳɰ���Ӧ�ﵽƽ��ı�ijһ������� ����
�ı���
��
����������Ӧ������ʱ��Ĺ�
ϵ����ͼ��ʾ��ͼ��ʱ����ƽ���ƶ�����������
�� �����б�ʾƽ���������ĺ�����
�ߵ�һ��ʱ���� ��
���¶�ΪT��ʱ����2a mol��a mol
����0��5 L �ܱ������У���ַ�Ӧ����
��ת����Ϊ50������÷�Ӧ��ƽ�ⳣ��Ϊ ��
��1���������ڣ���VIIA�� ��2�� 2Na+2H2O=2Na++2OH-+H2��
��3�� Cl2+Na2S=2NaCl+S�� ��4����ͬ ��5����ѹ t2-t3 4/a2
����:��

| A��X��Y��Z���ַ��ӵ��ȶ������� | B����������Ԫ�ع����γ����ֵ��� | C��X��Y��Z���ַ��ӵķе������� | D��X��Y��Z���ַ��Ӿ�Ϊ���Է��� |

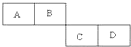 A��B��C��D���ֶ�����Ԫ�������ڱ��е����λ�������ʾ��A�ĵ�����ˮ������Ӧ������ȡˮú����
A��B��C��D���ֶ�����Ԫ�������ڱ��е����λ�������ʾ��A�ĵ�����ˮ������Ӧ������ȡˮú����