��Ŀ����
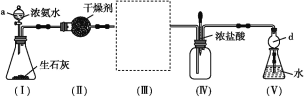
����Ŀ��ijʵ��С��ͬѧΪ�˲ⶨ��ҵ����Ĵ��ȣ�������һϵ��ʵ�顣
��1���������Ƽ��ȣ������Ƽ��������___________________________________��
��2����ҵ�����г���������NaCl���ʣ�����ԭ��__________________________�������Ƿ����Ȼ������ʵķ���Ϊ__________________________________________________________��
��3��ʹ���������ⶨ����Ĵ��ȣ��õ����Լ���__________________________________��
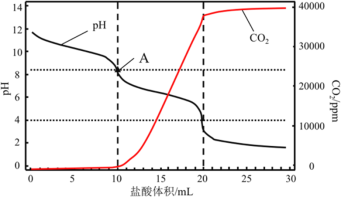
��4��ʹ�õζ����ⶨ����Ĵ��ȣ���_________________�����������ƣ�����1.200g��ҵ������Ʒ���ܽ⣬��1mol/L��������Һ���ζ���������ҺpH�仯������CO2������ͼ��ʾ��A����Һ�ʼ��Ե�ԭ��______________________________________________________������ù�ҵ������Ʒ����������_______________����������������λС����
 ��
��
���𰸡��Ȼ��������ʸߣ���ȾС���ɱ��͵ȣ�����������ɣ� �Ȼ������Ʊ������ԭ�ϣ���������̼�����ƾ����������Ȼ��Ʋ��� ȡ�����μӹ���ϡ�����ữ����������������������Һ�����۲쵽��ɫ�����������Ȼ������� CaCl2(BaCl2) ������ƽ A����ҺΪNaHCO3����Һ������HCO3-H++CO32-����ƽ���HCO3-+H2OH2CO+OH-ˮ��ƽ�⣬HCO3-�ĵ���С��ˮ�⣬������Һ�ʼ��� 0.88
��������
(1)�������ʳ�Σ��Ȼ��ƣ���ʯ��ʯ��������������ʯ�ҺͶ�����̼��������Ϊԭ������ȡ��������Ƽ����ʳ�Ρ����Ͷ�����̼�����ж�����̼���Ժϳɰ�����ˮú����ȡ����ʱ�ķ�����Ϊԭ������ȡ������ݷ�Ӧԭ��������ȱ�㣻
(2)NaCl����ȡ�����ԭ�����ݴ˷����������Ƿ����Ȼ������ʼ�Ϊ���������ӵĴ��ڣ�
(3)����̼���ơ��Ȼ��ƵĻ�ѧ���ʽ��з����жϣ�
(4)����1.200g��ҵ������Ʒ����Ҫ��ȷ�Ƚϸߵij��������� A ����ҺΪNaHCO3����Һ��̼��������ӿ�ˮ��Ҳ�ɵ��룬����ˮ��͵���̶ȷ����жϡ�
(1)������ܵĸ�����Ϊ�Ȼ��ƣ������Ƽ���ܵĸ������Ȼ�泥������Ƽ�백���ȣ����������Դ�����CaCl2��ͬʱ��������NH4Cl�����ʣ�ͬʱ�����ʳ�ε������ʣ��������Ƽ��ȣ���ȾС���ɱ��͵ȣ�
(2)�Ȼ������Ʊ������ԭ�ϣ���������̼�����ƾ����������Ȼ��Ʋ������ʹ�ҵ�����г���������NaCl���ʣ������Ƿ����Ȼ�������ֻ��Ҫ�����Ƿ��������Ӽ��ɣ�����Ϊȡ�����μӹ���ϡ�����ữ����������������������Һ�����۲쵽��ɫ�����������Ȼ������ʣ�
(3)����̼���ƵĻ�ѧ���ʣ�̼������ BaCl2��Һ(��CaCl2)��Һ��Ӧ�ֱ���̼�ᱵ��̼��ư�ɫ�������ɣ������ɳ��������������̼���Ƶ������������ɼ�����ù�ҵ����Ĵ��ȣ���ʹ���������ⶨ����Ĵ��ȣ��õ����Լ���CaCl2(��BaCl2)��
(4)ʹ�õζ����ⶨ����Ĵ��ȣ�����1.200g��ҵ������Ʒ��Ҫʹ�þ�ȷ�Ƚϸߵij���������Ӧ�õ�����ƽ��A��ʱ����1mol/L�����Һ���Ϊ10mL����ʱ��Һ��̼����ȫ��ת��Ϊ̼�����ƣ���A����ҺΪNaHCO3����Һ������HCO3-H++CO32-����ƽ���HCO3-+H2OH2CO+OH-ˮ��ƽ�⣬HCO3-�ĵ���С��ˮ�⣬������Һ�ʼ��ԣ�����ͼʾ����1mol/L�����Һ���Ϊ20mLʱ��̼����ȫ��ת��ΪCO2���ﵽ�ζ��յ㣬���ݷ�ӦNa2CO3+2HCl=2NaCl+H2O+CO2����������20mL����ʱ��n(Na2CO3)=![]() n(HCl)=
n(HCl)=![]() ��20mL��10-3��1mol/L=0.01mol���ù�ҵ������Ʒ����������=
��20mL��10-3��1mol/L=0.01mol���ù�ҵ������Ʒ����������=![]() =0.88��
=0.88��

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�����Ŀ�����ж�ʵ����ʵ�Ľ��ʹ�����ǣ� ��
ѡ�� | ʵ����ʵ | ���� |
A | ʵ�����ô�п��ϡ���ᷴӦ��H2�ȴ�п�� | ��п��ϡ���ṹ��ԭ��� |
B | Ũ���ᱣ������ɫ�Լ�ƿ�� | 4HNO3 |
C | ��10mL0.2mol��L-1ZnSO4����Һ�м���10mL0.4mol��L-1Na2S��Һ��������ɫ�������ٵμ�CuSO4��Һ��������� | Ksp(CuS)<Ksp(ZnS) |
D | ����������Ӧ���Թܿ���������Һϴ�ӣ�����ϡ���ᣬ��ϴЧ������ | Fe3++Ag |
A.AB.BC.CD.D