��Ŀ����
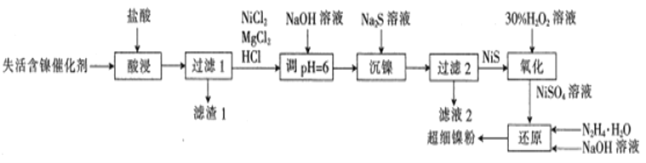
����Ŀ���й��Ը߷����ֳ�Ϊ�������Ը߷���������һ���ڲʵ�ӫ���������ģ���ɵ�·������Ӧ�ýϹ�����߷��Ӳ��ϣ���ṹ��ʽΪ ��
��
�Իش��������⣺
��1�������ʵĵ�����______��
��2����һ�������£��ø߾���ɷ����ķ�Ӧ��__________(�����)��
�ټӳɷ�Ӧ ��������Ӧ ��ȡ����Ӧ ��������Ӧ
��3���ø߾����ڴ����������£�ˮ���õ���Է���������С�IJ���ΪA����
��A�ķ���ʽ��_____��
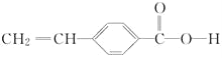
��A��һ�����������Ҵ���Ӧ�Ļ�ѧ����ʽ��________��
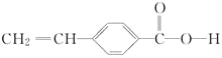
��A��ͬ���칹���ж��֣����к��б�����![]() ��
��![]() ���ұ�������������λȡ�����Ľṹ��ʽ��_______��______��
���ұ�������������λȡ�����Ľṹ��ʽ��_______��______��
���𰸡�![]() �٢ڢ� C9H8O2
�٢ڢ� C9H8O2 ![]() =CH��COOH��C2H5OH
=CH��COOH��C2H5OH![]()
![]() =CH��COOC2H5��H2O
=CH��COOC2H5��H2O 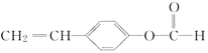
��������
�߷��Ӳ���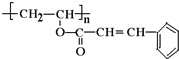 �ĵ���Ϊ
�ĵ���Ϊ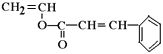 ��
��  �ڴ����������£�ˮ���õ�
�ڴ����������£�ˮ���õ�![]() ��
��![]() ����Ϲ����ŵ����ʷ������
����Ϲ����ŵ����ʷ������
(1)�߷��Ӳ��� �ĵ���Ϊ
�ĵ���Ϊ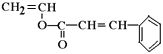 ���ʴ�Ϊ��
���ʴ�Ϊ��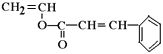 ��
��
(2)�߾��� �к���̼̼˫�����ɷ����ӳɡ��Ӿۺ�������Ӧ�������������ɷ���ˮ�ⷴӦ��ȡ����Ӧ�����б��������ڴ������±�±�ص���ȡ�����ʴ�Ϊ���٢ڢۣ�
�к���̼̼˫�����ɷ����ӳɡ��Ӿۺ�������Ӧ�������������ɷ���ˮ�ⷴӦ��ȡ����Ӧ�����б��������ڴ������±�±�ص���ȡ�����ʴ�Ϊ���٢ڢۣ�
(3)�߾��� �ڴ����������£�ˮ���õ�
�ڴ����������£�ˮ���õ�![]() ��
��![]() ��������Է���������С��Ϊ
��������Է���������С��Ϊ![]() ��
��
��AΪ![]() �������ʽΪC9H8O2���ʴ�Ϊ��C9H8O2��
�������ʽΪC9H8O2���ʴ�Ϊ��C9H8O2��
��AΪ![]() �������Ȼ��������Ҵ�����������Ӧ����Ӧ�Ļ�ѧ����ʽΪ
�������Ȼ��������Ҵ�����������Ӧ����Ӧ�Ļ�ѧ����ʽΪ![]() ��C2H5OH
��C2H5OH![]()
![]() =CH��COOC2H5��H2O���ʴ�Ϊ��
=CH��COOC2H5��H2O���ʴ�Ϊ��![]() ��C2H5OH
��C2H5OH![]()
![]() -CH=CH��COOC2H5��H2O��
-CH=CH��COOC2H5��H2O��
��A(![]() )��ͬ���칹���ж��֣����к��б�����
)��ͬ���칹���ж��֣����к��б�����![]() ��
��![]() ���ұ�������������λȡ�����Ľṹ��ʽ��
���ұ�������������λȡ�����Ľṹ��ʽ��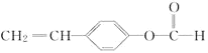 ��
�� ���ʴ�Ϊ��
���ʴ�Ϊ�� ��
�� ��
��

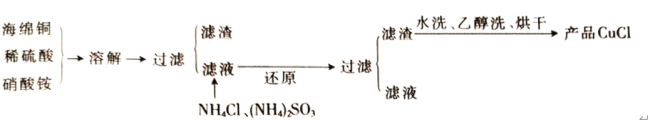
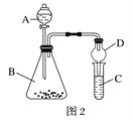
����Ŀ������ͭ��һ��Ӧ�ü���㷺�Ļ���ԭ�ϣ�������ij�����ú�����ͭΪԭ������������CuSO45H2O��������ʯ�ࣨCaSO42H2O������������ʾ��ͼ��
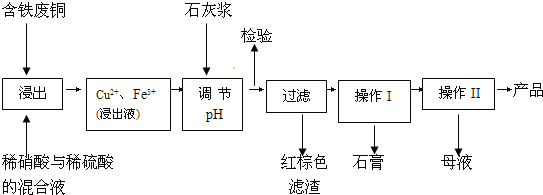
������ʯ���ڲ�ͬ�¶��µ��ܽ�ȣ�g/100gˮ��������
�¶ȣ��棩 | 20 | 40 | 60 | 80 | 100 |
ʯ�� | 0.32 | 0.26 | 0.15 | 0.11 | 0.07 |
���� | 32 | 44.6 | 61.8 | 83.8 | 114 |
��ش��������⣺
��1������ɫ��������Ҫ�ɷ���___��
��2��д��������������������ͭ�����ӷ���ʽ___��
��3������I��������Ũ����__�Ȳ������¶�Ӧ�ÿ�����__�����ң�
��4������Һ�з��������ͭ����IJ�����ӦΪ__��___��ϴ�ӡ������������ˮ�Ҵ���ϴ��Һ����������ˮ��ԭ����__������ʱ�������ɣ����ü��Ⱥ�ɵ�ԭ����___��
��5��ȡ��������Ϊ��ȷ��Fe3+�Ƿ��������ͬѧ������������ַ�������ʵ���ҷֱ����ȡ��Ʒ�����з������в�����
����һ��ȡ�����Թ����μ�KSCN��Һ�� ��������ֽ��������KSCN��Һ��
������Ϊ�������ַ�����Ƹ���������__��
��ָ������Ϊ�������������ڵ�������__��