��Ŀ����
����������Ԫ��A��B��C��D��ԭ��������������A��B��Cԭ�ӵ�����������֮��Ϊ12��B��C��Dλ��ͬһ���ڣ�Cԭ�ӵ���������������Aԭ���ڲ��������3������Bԭ��������������3��������˵����ȷ����
- A.Ԫ��A��C������������Ӧ��ˮ���ﶼ������
- B.Ԫ��B����A�������������û���Ӧ
- C.Ԫ��B��D���γ�BD2�͵Ĺ��ۻ�����
- D.D�ĵ����ж�������Ư����
B
B��C��Dλ��ͬһ���ڣ�Cԭ�ӵ���������������Aԭ���ڲ��������3������Bԭ��������������3������˵��B��þ��C������A��̼��D���ȡ�A����ȷ��������ǿ�ᣬþ������CO2��ȼ�գ���������þ�͵���̼��B��ȷ���Ȼ�þ�����ӻ����C����ȷ������û��Ư���ԣ�����ˮ���ɵĴ��������Ư���ԣ���ѡB��
B��C��Dλ��ͬһ���ڣ�Cԭ�ӵ���������������Aԭ���ڲ��������3������Bԭ��������������3������˵��B��þ��C������A��̼��D���ȡ�A����ȷ��������ǿ�ᣬþ������CO2��ȼ�գ���������þ�͵���̼��B��ȷ���Ȼ�þ�����ӻ����C����ȷ������û��Ư���ԣ�����ˮ���ɵĴ��������Ư���ԣ���ѡB��

��ϰ��ϵ�д�
 ���Ž�������С״Ԫϵ�д�
���Ž�������С״Ԫϵ�д�
�����Ŀ
����������Ԫ��A��B��C��D��ԭ��������������A��Cԭ���������8��A��B��C����Ԫ��ԭ�ӵ�����������֮��Ϊ15��Bԭ����������������Aԭ��������������һ�룮����������ȷ���ǣ�������
| A��ԭ�Ӱ뾶��A��D��C��B | B��B��C��D�ֱ���A�γɵĻ�����һ��������ͬ�Ļ�ѧ�� | C������������Ӧˮ��������ԣ�D��C | D�������£�����B�ܴ�������Ũ������ |
����������Ԫ��A��B��C��ԭ���������ε�����A��Cͬ���壬Bԭ�ӵ���������������Aԭ�ӵĴ���������������ԭ�ӵ�����������֮��Ϊ10��������������ȷ���ǣ�������
| A��ԭ�ڰ뾶A��B��C | B��A����̬�⻯���ȶ��Դ���C����̬�⻯���ȶ��� | C��A��C��Ԫ����������������ˮ���ϵõ���Ӧ���� | D������ʱ��A���ʿ��Դ�C�����������û��õ�C���� |
����������Ԫ��A��B��C��D��E��ԭ������������������A��Cͬ���壬A������Ԫ�ز���ͬһ���ڣ�B��Dͬ���壬������D�ĵ���Ϊ����ɫ���壮�����ƶ�����ȷ���ǣ�������
| A��ԭ�Ӱ뾶��С�����˳��r��C����r��D����r��E�� | B��Ԫ��D��E�ֱ���A�γɵĻ���������ȶ��ԣ�E��D | C��Ԫ��D������������Ӧˮ��������Ա�E��ǿ | D��Ԫ��B�ֱ���A��C�γɵĻ������л�ѧ����������ȫ��ͬ |
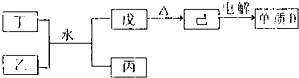
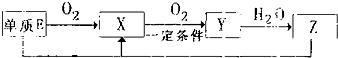
 ����������Ԫ��A��B��C��D��E��ԭ������������������ԭ�Ӻ���ĵ��Ӳ���֮��Ϊ10��BԪ�صĻ���������࣬��Ŀ�Ӵ�C��D����Ԫ���γɵĵ����ǿ����к����������ʣ�D��E��Ԫ�ؿ����������ֲ�ͬ�����ӻ����
����������Ԫ��A��B��C��D��E��ԭ������������������ԭ�Ӻ���ĵ��Ӳ���֮��Ϊ10��BԪ�صĻ���������࣬��Ŀ�Ӵ�C��D����Ԫ���γɵĵ����ǿ����к����������ʣ�D��E��Ԫ�ؿ����������ֲ�ͬ�����ӻ���� NH3?H2O+H+
NH3?H2O+H+ 2NO2
2NO2