��Ŀ����
����Ŀ���ҹ����ຣʡ�������κ�ʢ��ʳ�Σ�������ʳ�ι�ϵ���У�ʳ�����ϰ���������ִ����Ĺ�ũҵ�����о�����Ҫ���á������к�Ca2+��Mg2+��SO42-�Լ���ɳ�����ʣ�Ϊ�˳�ȥ���������ʣ�������ʵ�鲽������ᴿ��
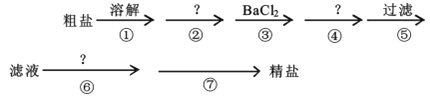
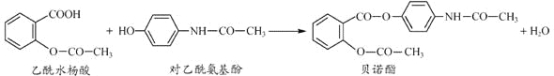
(1)�ܲ������Լ���___��
(2)�ڢ�������Ӧ�����ӷ���ʽΪ___��___��
(3)�ڢ߲��IJ�������___����Ҫ�IJ���������___��___��
(4)ͨ��������й��˺����Һ������SO42-�ѳ����IJ���������___��
���𰸡�Na2CO3��Һ H++OH-=H2O 2H++CO32-=CO2����H2O �����ᾧ �ƾ��� ������ ȡ��������Һ���Թ��У������μ�BaCl2��Һ����û������˵��SO42-�ѳ���
��������
�����к�Ca2+��Mg2+��SO42-�Լ���ɳ�����ʣ��ڴ����ᴿʱ��̼������ҺҪ���Ȼ�����Һ�ĺ�����룬�ɳ�ȥ�����Ӻͼ���Ĺ����ı����ӣ�NaOH��Һ�ܹ���þ���ӣ����˺��ٽ�һ���������ᣬ��ȥ���������������Ӻ�̼������ӣ��õ�NaCl��Һ�������ᾧ�õ�NaCl���壬������̣����Ӧ�������NaOH��Һ��þ���ӣ��ܲ������Լ���̼���ƣ���ȥ�����Ӻͼ���Ĺ����ı����ӣ��������ᣬ��ȥ���������������Ӻ�̼������ӣ��������ᾧ���ݴ˷������
(1)�ɷ�����֪�ܲ������Լ���Na2CO3��Һ��Ŀ���dz�ȥ�����Ӻͼ���Ĺ����ı����ӣ��ʴ�Ϊ��Na2CO3��Һ��
(2)�ɷ�����֪�ڢ��������ᣬ��ȥ���������������Ӻ�̼������ӣ�������Ӧ�����ӷ���ʽΪH++OH-=H2O��2H++CO32-=CO2��+H2O���ʴ�Ϊ��H++OH-=H2O��2H++CO32-=CO2��+H2O��
(3)�ɷ�����֪�ڢ߲��IJ������������ᾧ����Ҫ�IJ��������оƾ��ơ����������ʴ�Ϊ�������ᾧ���ƾ��ƣ���������
(4)ͨ��������й��˺����Һ������SO42-�ѳ����IJ���������ȡ��������Һ���Թ��У����������Һ�еμ�BaCl2��Һ����û������˵��SO42-�ѳ������ʴ�Ϊ��ȡ��������Һ���Թ��У������μ�BaCl2��Һ����û������˵��SO42-�ѳ�����

����Ŀ��(һ)���������õ������£�NH4+����������Ӧ�������� NO3-��������Ӧ�������仯ʾ��ͼ���£�
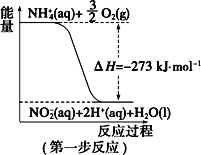
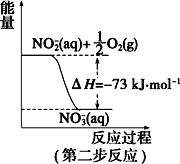
(1)��һ����Ӧ��_________(��������������������)��Ӧ��
(2)1 mol NH4+ (aq)ȫ��������NO3- (aq)���Ȼ�ѧ����ʽ��___________��
(��)�������(Fe)ȥ��ˮ���е�������(NO3-)�ѳ�Ϊ�������о����ȵ�֮һ��
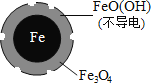
(1)Fe��ԭˮ����NO3-�ķ�Ӧԭ����ͼ��ʾ��
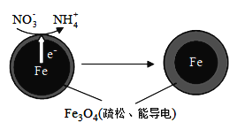
�������������ʻ�ѧʽΪ___________��
�������ĵ缫��Ӧʽ��_______________��
(2)����������Ͷ��ˮ���У���24Сʱ�ⶨNO3-��ȥ���ʺ�pH��������£�
��ʼpH | pH=2.5 | pH=4.5 |
NO3-��ȥ���� | �ӽ�100% | ��50% |
24СʱpH | �ӽ����� | �ӽ����� |
��������������̬ |
|
|
pH=4.5ʱ��NO3-��ȥ���ʵ͡���ԭ����_______________��
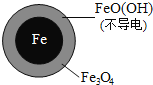
(3)ʵ�鷢�֣��ڳ�ʼpH=4.5��ˮ����Ͷ���������۵�ͬʱ������һ������Fe2+�����������NO3-��ȥ���ʡ���Fe2+������������ּ��裺
��Fe2+ֱ�ӻ�ԭNO3-��
��Fe2+�ƻ�FeO(OH)�����㡣
�����Ա�ʵ�飬�����ͼ��ʾ���ɵõ��Ľ�����_________��

��ͬλ��ʾ�ٷ�֤ʵFe2+����FeO(OH)��Ӧ����Fe3O4���÷�Ӧ�����ӷ���ʽΪ_________���ͼ���Fe2+���NO3-ȥ���ʵ�ԭ��________��