��Ŀ����
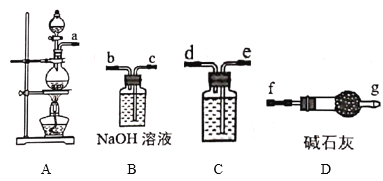
����Ŀ���ö��Ե缫���һ����������ͭ��Һ��ʵ��װ����ͼ�ס��������е�ʵ��������ͼ�ң��������ʾ��������ת�Ƶ��ӵ����ʵ������������ʾ�������в�����������������״������������˵������ȷ����

A.�������У�a�缫�������к�ɫ�����������������ݲ���
B.b�缫�Ϸ����ķ�Ӧ����ʽΪ��4OH��һ4e����2H2O+O2��
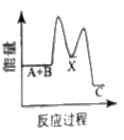
C.����OһP�α�ʾH2������仯
D.Q��ʱ�ռ����Ļ��������H2��O2�����Ϊ1��1
���𰸡�C
��������
�������ͭ��Һʱ�������ɵ�ص�������������bΪ������aΪ�������缫��Ӧʽ��������2H2O-4e����4H++ O2����������Cu2++ 2e����Cu��2H++ 2e����H2����
A. �������У�a�缫Ϊ��������Һ�е�Cu2+�õ�������Cu����������к�ɫ��������������H+�õ������������������ݲ�����A��ȷ��
B. b�缫Ϊ��������Һ�е�ˮʧ�������������������ķ�Ӧ����ʽΪ��4OH��-4e����2H2O+O2����B��ȷ��
C. ����O-P�Σ���������������������Cu����ʾO2������仯��C����
D. Q��ʱ�ռ����Ļ�������У�H2��O2����µ�����ֱ�Ϊ2.24L�������Ϊ1��1��D��ȷ��
��ΪC��

 ��ĩ�����ϵ�д�
��ĩ�����ϵ�д�����Ŀ������������Ϊһ�����ʹ��ܵ�أ���Ӧ���õ����Ӻͷ�չ�������������ڽ����ơ�������Ͷ����ƣ�Na2Sx���ֱ���Ϊ�����缫�ķ�Ӧ�����Al2O3�մɣ��ɴ���Na+��Ϊ����ʣ��䷴Ӧԭ����ͼ��ʾ��
���� | Na | S | Al2O3 |
�۵�/�� | 97.8 | 115 | 2050 |
�е�/�� | 892 | 444.6 | 2980 |
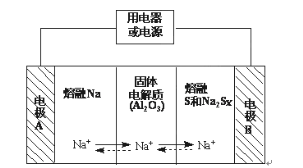
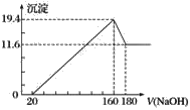
��1�����жϸõ�ع����������¶�Ӧ������____��Χ�ڣ�����ĸ��ţ���
A.���� B.60�桫 100�� C.200�桫350�� D.2000�桫3000��
��2���ŵ�ʱ���缫AΪ___����
��3���ŵ�ʱ���ڵ�·��Na+���ƶ�����Ϊ___��������A��B��������B��A������
��4�����ʱ���ܷ�ӦΪNa2Sx��2Na+xS��3<x<5�����������ĵ缫��ӦʽΪ___��
����NaOH��Һ���������е�SO2�������õ�Na2SO3��Һ���е�⣬��ѭ������NaOH��ͬʱ�õ�H2SO4����ԭ����ͼ��ʾ�����缫����Ϊʯī��

��1��ͼ��a��Ҫ���ӵ�Դ�ģ�������������������___����C��������������___��
��2��SO32���ŵ�ĵ缫��ӦʽΪ____��
��3��������������������������ǿ�����ϵ缫����ʽ����ԭ��___��