��Ŀ����
����Ŀ��I����A��B��C��D��E���ֶ���������Ԫ�أ����ǵ�ԭ��������������AԪ�ص�ԭ���ǰ뾶��С��ԭ�ӣ�BԪ�ص�����������ˮ���������⻯�ﷴӦ����һ����X��D��Aͬ�壬����Eͬ���ڣ�EԪ�ص������������Ǵ�����������3/4����A��B��D��E������Ԫ�أ�ÿһ�ֶ�����CԪ���γ�ԭ�Ӹ����Ȳ���ͬ�Ķ��ֻ�����ش��������⣺
��1��д����ӦԪ�����ƣ� C_________
��2��������ס��Ҿ�����A��C��D��E����Ԫ����ɵ���ѧ�����Ļ�������������ᷴӦ��������NaOH��Һ��Ӧ������Һ������Һ��Ӧ�����ӷ���ʽΪ��_____________________������C��D��E����Ԫ����ɣ�ԭ�Ӹ�����Ϊ3��2��2��������Һ�ͱ���Һ��Ӧ�����ӷ���ʽΪ��_________________��
��3��N2H4�ĵ���ʽΪ________����N2H4��O2ͨ�뵽��A��C��D����Ԫ��������ʵ�ϡ��Һ�й���ԭ��أ����ĵ缫��ӦʽΪ_______________��
�� ��֪X��һ���Σ�H�dz����������ʣ�F��I�dz����ǽ������ʣ�E��G���ǹ�ҵ����Ҫ�ļ������ʣ���������ͼ��ʾ�Ĺ�ϵ��
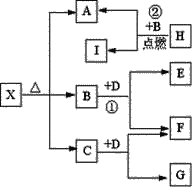
�Իش���������
��1��G�к��еĻ�ѧ�������У�_________________��
��2��д���ڷ�Ӧ�Ļ�ѧ����ʽ__________________����˫���ŷ���ʾ�÷�Ӧ����ת�Ƶķ������Ŀ_________________��
���𰸡�����1��CΪ�� ����2��HSO32����H���� SO2����H2O
S2O32����2H����S���� SO2����H2O ��3��![]() ��1�֣�N2H4-4e-+4OH- =N2+4H2O
��1�֣�N2H4-4e-+4OH- =N2+4H2O
����1�����Ӽ������ۼ� ��2��2Mg + CO2![]() 2MgO + C
2MgO + C
��������
������ AԪ�ص�ԭ���ǰ뾶��С��ԭ�ӣ���AΪ��ԭ�ӣ�BԪ�ص�����������ˮ���������⻯�ﷴӦ����һ����X��BΪ��Ԫ�ء�XΪ����泥�EԪ�ص������������Ǵ�����������3/4����EΪ��Ԫ�أ�D��Aͬ�壬����Eͬ���ڣ�����DΪ��Ԫ�أ�A��B��D��E������Ԫ�أ�ÿһ�ֶ�����CԪ���γ�ԭ�Ӹ����Ȳ���ͬ�Ķ��ֻ����CΪ��Ԫ�أ���1��CΪ������2��������ס��Ҿ�����A��C��D��E����Ԫ����ɵ���ѧ�����Ļ�������������ᷴӦ��������NaOH��Һ��Ӧ�����Լ�Ϊ![]() ����Ϊ
����Ϊ![]() ������Һ������Һ��Ӧ�����ӷ���ʽΪHSO32����H���� SO2����H2O������C��D��E����Ԫ����ɣ�ԭ�Ӹ�����Ϊ3��2��2�������Ա�Ϊ
������Һ������Һ��Ӧ�����ӷ���ʽΪHSO32����H���� SO2����H2O������C��D��E����Ԫ����ɣ�ԭ�Ӹ�����Ϊ3��2��2�������Ա�Ϊ![]() ������Һ�ͱ���Һ��Ӧ�����ӷ���ʽΪS2O32����2H����S���� SO2����H2O ����3��N2H4�ĵ���ʽΪ
������Һ�ͱ���Һ��Ӧ�����ӷ���ʽΪS2O32����2H����S���� SO2����H2O ����3��N2H4�ĵ���ʽΪ![]() ����N2H4��O2ͨ�뵽��A��C��D����Ԫ��������ʵ�ϡ��Һ�й���ԭ��أ�ͨ��N2H4��һ��Ϊ���������ĵ缫��ӦʽΪN2H4-4e-+4OH- =N2+4H2O��
����N2H4��O2ͨ�뵽��A��C��D����Ԫ��������ʵ�ϡ��Һ�й���ԭ��أ�ͨ��N2H4��һ��Ϊ���������ĵ缫��ӦʽΪN2H4-4e-+4OH- =N2+4H2O��
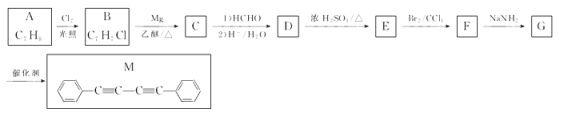
���ӷ�Ӧ������������һ�������û��ǽ������û���Ӧ��������Ϊ��ȼ��Ӧ���뵽2Mg+CO2![]() 2MgO+C�����Ӧ���Ӷ���֪BΪCO2��xΪ�Σ��ηֽ�IJ���һ����ˮ������x�ֽ��õ�MgO��CO2��H2O���Ӷ��Ƴ�xΪ��ʽ̼��þ��A��MgO��B��CO2��C��H2O;D��Na2O2��E��Na2CO3��F��O2;G��NaOH��H��Mg ����Ӧ���Ļ�ѧ����ʽ��:2Mg+CO2
2MgO+C�����Ӧ���Ӷ���֪BΪCO2��xΪ�Σ��ηֽ�IJ���һ����ˮ������x�ֽ��õ�MgO��CO2��H2O���Ӷ��Ƴ�xΪ��ʽ̼��þ��A��MgO��B��CO2��C��H2O;D��Na2O2��E��Na2CO3��F��O2;G��NaOH��H��Mg ����Ӧ���Ļ�ѧ����ʽ��:2Mg+CO2![]() 2MgO+C;(3)��ʽ��X�����ᷴӦ�Ļ�ѧ����ʽΪMg4(OH)2(CO3)3+8HCl=4MgCl2+3CO2��+5H2O���� 1 ��NaOH�к��еĻ�ѧ�����������Ӽ������ۼ�����2������Ӧ�Ļ�ѧ����ʽ2Mg + CO2
2MgO+C;(3)��ʽ��X�����ᷴӦ�Ļ�ѧ����ʽΪMg4(OH)2(CO3)3+8HCl=4MgCl2+3CO2��+5H2O���� 1 ��NaOH�к��еĻ�ѧ�����������Ӽ������ۼ�����2������Ӧ�Ļ�ѧ����ʽ2Mg + CO2![]() 2MgO + C���÷�Ӧ����ת�Ƶķ������Ŀ
2MgO + C���÷�Ӧ����ת�Ƶķ������Ŀ ��
��

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�����Ŀ���ڹŴ����ٺ�ɫ��Ǧ����Pb3O4��������ҩ�����������Ƕ������ؽ���Ǧ�Ķ�����ʶ���㡣��֪��PbO2Ϊ�غ�ɫ��ĩ��ij��ѧ��ȤС���Ǧ����һЩ���ʽ���ʵ��̽�����ⶨ����ɡ�
�ش��������⣺
��1������ʵ��
ʵ����� | ���� | ���ͻ���� |
�ٽ�����Ǧ����Ʒ����С�ձ��У�����2mL6mol/L��HNO3��Һ������ | _____ | Pb3O4��4HNO3=PbO2�� 2Pb��NO3��2��2H2O |
�ڽ������������ˣ�����������Ϊ���ݣ�һ�ݼ���2mLŨ���ᣬ���� | �д̼��ԵĻ���ɫ������� | ��Ӧ�Ļ�ѧ����ʽ�� _______ |
����һ���������������ữ��Mn��NO3��2��Һ������ | ����ɫ��Һ | ���ۣ�_______ |
��2����ɲⶨ
��ȷ��ȡ0.530g�����Ǧ����Ʒ�����ڽྻ��С�ձ��У�����2mL6mol/L��HNO3��Һ������ʹ֮��ַ�Ӧ��������������Һ���÷������������_____________��
��������������Һȫ��ת����ƿ�У�����ָʾ���ͻ�����Һ����0.04000mol/L��EDTA��Һ�������ԣ��ζ����յ㣬����EDTA��Һ36.50mL��EDTA��Pb2+�ķ�Ӧ�ɱ�ʾΪPb2+��H2Y2-=PbY2-��2H+���ζ�ʱEDTA��ҺӦʢװ��_______________�С���Һ�к�Pb2+__________mol��
�����������ù���PbO2ȫ��ת����һ��ƿ�У������м�������HAc��NaAc�Ļ��Һ��8g���� KI��ҡ����ƿ��ʹPbO2ȫ����Ӧ���ܽ⣬������ӦPbO2��4I����4HAc =PbI2��I2��4Ac����2H2O����ʱ��Һ������ɫ����0.05000mol/L��Na2S2O3����Һ�ζ���������ӦI2��2S2O32-=S4O62-��2I��������Һ�ʵ���ɫʱ����2%������Һ1mL�������ζ�����Һ_______����Ϊ�յ㣬��ȥNa2S2O3��Һ30.80mL��
���ݢڡ���ʵ�����ݼ��㣬Ǧ����Pb������Pb��������ԭ����֮��Ϊ____________��